AS Level Chemistry 9701
4. States of Matter
Written by: Adhulan R
Formatted by: Rithanya S
4.1 The gaseous state
Kinetic theory of gases
- Idea that molecules in a gas are in constant movement is called the kinetic theory of gases
- This theory makes certain assumptions:
- Gas molecules move rapidly and randomly
- Gas molecules are far apart, so their volume is negligible (Zero particle volume)
- No intermolecular forces exist between gas molecules
- Collisions are elastic, with no loss of kinetic energy
- Gas temperature is proportional to the average kinetic energy of molecules
- A theoretical gas that fit all these assumptions is called an ideal gas
- Pressure in gas → Collision of gas particles with the walls of the container
Ideal gas equation
- The volume occupied by gas depends on:
- Its pressure (Pa)
- Its temperature (K)
- An ideal gas will have a volume that is exactly proportional to temperature and inversely proportional to pressure
- Gases don’t obey the ideal gas laws completely (especially in low temp. and high pressure)
- There is not zero attraction b/w molecules
- We cannot ignore the volume of molecules even if small
- The general gas equation → pV = nRT
- p → pressure (Pa)
- V → volume (m3) [1 m3 = 1000 dm3]
- n → number of moles of gas (mol)
- R → ideal gas constant = 8.31 J K-1 mol-1
- T → temperature (K)
Measuring relative molecular mass (Mr)
- The number of moles of a substance is its mass / Mr
- Hence by finding the number of moles and mass of a substance using the ideal gas equation – we can find Mr
- Though measuring the mass of air is difficult, this can give approximate yet reasonable results
- Method can also be used for volatile liquids – involves syringe oven
5.2 Bonding and structure
- The state of a structure in room and temp. and pressure depends on its structure and bonding.
- Four type of structures:
- Simple molecular or simple atomic (Eg: Carbon dioxide, argon)
- Noble gases, despite existing as isolated atoms, are considered to have a simple molecular structure since they share similar physical properties with simple molecular gases.
- Giant ionic (Eg: Sodium Chloride)
- Giant metallic (Eg: Iron, Copper)
- Giant molecular (Eg: Silicon(IV) Oxide)
- Simple molecular or simple atomic (Eg: Carbon dioxide, argon)
- Crystal lattice → Regularly repeating arrangements of ions, atoms, or molecules
- Many ionic, metallic, and covalent compounds are crystalline
Giant ionic lattices
- 3-dimensional arrangement of alternating +ve and -ve ions
- Type of lattice formed depends on the relative size of the ions present
- Sodium chloride and magnesium oxide are cubic
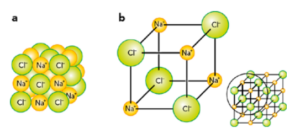
- Properties:
- Hard → Strong electrostatic forces
- Brittle → When struck in a specific direction, layers of ions may shift, aligning ions of the same charge. This causes electrostatic repulsion, leading to splitting along cleavage planes.
- High boiling point and melting point → Electrostatic forces acting in all directions – strongly bonds all ions together
- Many are soluble in water – as they can form ion-dipole bonds
- Conduct electricity when molten or in aqueous form
Giant metallic lattice
- Lattice contain ions surrounded by sea of electrons
- Ions are often packed in hexagonal layers or cubic arrangements

- When a force is applied → layers can slide over each other
- When layers slide, new metallic bonds are easily re-formed b/w ions in and delocalized electrons
- The delocalized electrons continue to hold ions together
- Properties:
- Malleable (layers can slide)
- Ductile (layers can slide)
- High tensile strength and hardness → strong metallic bond b/w electrons and ions
- When a force is applied → layers can slide over each other
Simple molecular lattice
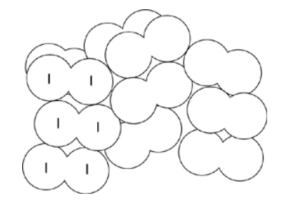
- Ice also forms a crystalline lattice → due to hydrogen bonding
- Intermolecular forces between iodine molecules are weak, while covalent bonds within iodine molecules are strong → low melting point
Buckminsterfullerene (C60)
- Fullerene structure based on rings of carbon atoms, just like in graphite
- Fullerenes are allotropes of carbon in the form of hollow spheres or tubes
- First fullerene discovered → Buckminsterfullerene, C60
- The carbon atoms are arranged in the corners of 20 hexagons and 20 pentagons

- Some electrons are delocalised (but lesser than graphite)
- Properties:
- Relatively low sublimation point → Weak intermolecular forces
- Relatively soft → Weak intermolecular forces
- Poor conductor of electricity relative to graphite → extent of electron delocalization is lower
- Slightly soluble
- More reactive than graphite and diamond
Giant molecular lattice
- Have a network of covalent bonds → high m.p. and b.p.
- Allotropes: different crystalline or molecular forms of the same element
- Diamond, graphite, buckminsterfullerene → allotropes of carbon
Graphite
- Atoms arranged in planar layers
- Within each layer – arranged in hexagons

- Each carbon atom joined with 3 other carbon atoms
- Fourth electron occupies p-orbital
- This p-orbital overlap sideways (b/w different planar layers)
- Clouds of delocalized electrons above and below the carbon rings
- These clouds join to form extended delocalised rings of electrons
- Layers held together by weak instantaneous dipole- induced dipole forces
- Properties:
- High m.p. and b.p. → Strong covalent bonds
- Softness → Easily scratched – bonds b/w layers are weak – layers can slide over each other (this leads to flakiness)
- Good conductor → Large clouds of delocalized electrons
Diamond
- Each carbon bonded with four other carbon

- Properties:
- High m.p. and b.p. → Strong covalent bonds
- Hardness → Difficult to break the 3-dimensional network of strong covalent bonds
- Does not conduct electricity → All 4 valence electrons of carbon are involved bonding
Silicon(IV) Oxide
- There are several forms of Silicon(IV) Oxide
- The SiO2 found in mineral quartz – similar to diamond (similar properties too)
- Each Silicon atom is bonded to 4 other oxygen atoms

