AS Level Chemistry 9701
3. Chemical Bonding
Written by: Pranav I
Formatted by: Rithanya S
7.1 Reversible reactions and equilibrium
- Reversible reaction: A reaction in which products can be changed back to reactants by reversing the conditions (have forward and backward reactions)
- Equilibrium reaction: A reaction that does not go to completion and in which reactants and products are present in fixed concentration ratios
Characteristics of equilibrium
- It is dynamic
- Reactants and products are continuously reacting → dynamic equilibrium
- The rate of forward and backward reactions are equal
- Reactants are being converted to products at the same rate as products are being converted back to reactants
- The concentrations of reactants and products remain constant at equilibrium
- Equilibrium requires a closed system
- Closed system: None of the reactants or products escape from the reaction mixture
7.2 Changing the position of equilibrium
Position of equilibrium
- Conc. of reactants > conc. of products → Position of equilibrium is to the left
- Conc. of products > conc. of reactants → Position of equilibrium is to the right
- The position of equilibrium can shift when the system is disturbed
Le Chatelier’s principle
- If a change is made to a system at dynamic equilibrium, the position of equilibrium moves to minimise this change
How does change in concentration affect the position of equilibrium?
- When the concentration of reactants increases, the position of equilibrium shifts to the right (more products formed to restore equilibrium)
- When the concentration of products increases, the position of equilibrium shifts to the left (more reactants formed to restore equilibrium)
The effect of pressure on the position of equilibrium
- Only affects reactions where gases are reactants or products
- When the pressure is increased:
- Molecules are closer together
- Position of equilibrium shifts to minimise this increase
- Shifts in the direction of fewer gas molecules → to oppose the increase in pressure
- More of the substance(s) in the side with fewer gas molecules is/are formed until equilibrium is restored
- The stoichiometric equation can be used to predict the effect
- Equal number of gas molecules on either side of the equation → change in pressure has no effect on the position of equilibrium
- In a reaction with substances in different states, only the gas molecules count
The effect of temperature on the position of equilibrium
- When the temperature is increased:
- The energy of the surroundings increases
- The reaction will go in the direction that opposes the increase in energy, as per the Le Chatelier’s principle
- The reaction will go in the direction in which energy is absorbed (endothermic)
- When the temperature is decreased:
- The energy of the surroundings decreases
- The reaction will go in the direction that opposes the decrease in energy
- The reaction will go in the direction in which energy is released (exothermic)
Do catalysts have any effect on the position of equilibrium?
- Catalysts ONLY reduce the time taken to reach the equilibrium
- They have no effect on the position of equilibrium
- They increase the rate of the forward and backward reactions equally
7.3 Equilibrium expressions and the equilibrium constant, Kc

Equilibrium expressions
- Equilibrium constant: A constant calculated from the equilibrium expression for a reaction
- Equilibrium expression: A simple relationship that links Kc to the equilibrium concentrations of reactants and products and the stoichiometric equation


- The unit of Kc depends on the form of the equilibrium expression (unit of concentration: mol dm-3)
- Kc and concentration changes
- Kc does not change when the concentration of reactants or products is altered
- Equilibrium is restored when the ratio of concentrations of products to reactants changes
- Kc and pressure changes
- Kc does not change when pressure is altered
- Kc and temperature changes
- Endothermic reaction → Kc increases as temperature increases
- Exothermic reaction → Kc decreases as temperature increases
7.4 Equilibria in gas reactions: the equilibrium constant, Kp
Partial pressure
- Partial pressure: The pressure exerted by a particular gas in a mixture of gases
- Total pressure is the sum of the partial pressures of individual gases
Equilibrium expressions involving partial pressures
- Kp → Equilibrium constant in terms of partial pressure
- The unit of Kp depends on the form of the equilibrium expression (unit of pressure: Pa)
Partial pressure and mole fractions
- Mole fraction: The number of moles of a particular gas in a mixture divided by the total number of moles of all the gases in the mixture
- Partial pressure of a gas is proportional to its concentration
- Partial pressure = Mole fraction x Total pressure
- Note: Sum of mole fractions = 1; sum of partial pressures = total pressure
7.5 Equilibria and the chemical industry
Equilibrium and ammonia production
- Haber process → the synthesis of ammonia
N2 + 3H2 ⇌ 2NH3 - Pressure is increased
- The position of equilibrium shifts to the right since it has fewer gas molecules → yield of ammonia increases
- To reduce pressure and counteract the change
- Temperature is decreased
- Favours the exothermic reaction that releases energy
- The position of equilibrium shifts to the right
- The value of Kp increases
- Removing ammonia by condensing it to a liquid
- Ammonia has a higher boiling point than H2 and N2
- The position of equilibrium shifts to the right to replace the ammonia that has been removed
Equilibrium and the production of sulfuric acid
- Contact process → synthesis of sulfuric acid
2SO2 + O2 ⇌ 2SO3 - Pressure is increased
- The position of equilibrium shifts to the right to reduce the pressure → fewer gas molecules produced
- The reaction has a very high Kp value
- Carried out just above atmospheric pressure
- Very high pressure is unnecessary and expensive
- Temperature is decreased
- Favours the exothermic reaction that releases energy
- The position of the equilibrium shifts to the right → the value of Kp increases
- SO3 is removed by absorbing it in 98% sulfuric acid
- SO3 reacts with the solution to eventually form more H2SO4
- The absorbing of SO3 does not affect the position of equilibrium since it is already far over to the right
7.6 Acid-base equilibria
pH values and neutralisation
- pH values can be deduced using a universal indicator or a pH metre
- Depends on the concentration of hydroxonium ions in a solution
- Neutralisation: The reaction of an acid with an alkali to form a salt and water
Acid + Alkali → Salt + Water
(H+ + OH– → H2O)
Some simple definitions of acids and bases
- Acid: A substance that neutralises a base
- Base: A substance that neutralises an acid
- A base is a compound that contains oxide or hydroxide ions and reacts with an acid to form a salt and water
- Alkalis are bases which are soluble in water
- Note: Ammonia is an alkali (forms NH4+ and OH– ions when dissolved in water)
The Bronsted-Lowry theory of acids and bases
- Bronsted-Lowry Acid: A proton (H+ ion) donor
- Bronsted-Lowry Base: A proton (H+ ion) acceptor

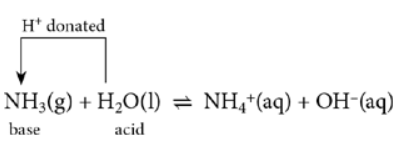
- Hydroxonium ion → H3O+
- Substances which can act as either acids or bases are amphoteric (Eg: H2O)
- When an acid or base reacts with water, an equilibrium mixture is formed
- In the case of HCl, the position of equilibrium is entirely in favour of the products (goes to completion)
HCl + aq → H+ + Cl– - For ammonia, the position of equilibrium favours the reactants
NH3 + H2O ⇌ NH4+ + OH–
- In the case of HCl, the position of equilibrium is entirely in favour of the products (goes to completion)
Strong and weak acids and bases
- Weak and strong refers to the degree of dissociation and not concentration
- Strong acids: Acids that fully dissociate in aqueous solutions (Eg: Mineral acids, HCl, H2SO4 and HNO3)
- Weak acids: Acids that partially dissociate in aqueous solutions (Eg: Organic acids, hydrocyanic acid, hydrogen sulphide and carbonic acid)

- Strong bases: Bases that fully dissociate in aqueous solutions (Eg: Group I hydroxides)
- Weak bases: Bases which partially dissociate in aqueous solutions (Eg: ammonia, amines and some hydroxides of transition metals)
Distinguishing a weak acid from a strong acid
- Note: Dilute solutions of the same concentration have to be used for the comparisons
- pH values
- The concentration of hydrogen ions is greater in strong acids
- Have lower pH values than weak acids
- Electrical conductivity
- The concentration of hydrogen ions and other ions is greater in strong acids
- Have greater electrical conductivity than weak acids
- Dip a conductivity electrode into a solution of the acid → connect a conductivity metre to the electrode
- Reaction with reactive metals
- Strong acids have a higher reactivity with reactive metals while compared to weak acids
- Due to the greater concentration of hydrogen ions
7.7 Indicators and acid-base titrations
Introducing indicators
- Acid-base indicator: A dye or mixture of dyes that changes colour over a specific pH range of between 1 and 2 pH units
- Can be considered as weak acids → acid and conjugate base have different colours
🔥 Important
An acid and base on different sides of the equation are said to be conjugate with each other if one can be converted to the other by gain or loss of a hydrogen ion.
HIn (un-ionised indicator → colour A) ⇌ H+ + In– (conjugate base → colour B)
- The colour shown by the indicator depends on the concentration of H+ ions
- Adding an acid → shifts the position of equilibrium to the left (increase in the concentration of one of the products) based on H+ ions conc.
- Adding an alkali → shifts the position of equilibrium to the right
- In the middle of the range, there is a recognisable end-point → indicator has a colour in between the two extremes of colour
Strongs acids with strong bases

- Sharp pH change over such a wide pH range (eg: pH 6.5 to pH 10.5)
- Thus, many indicators can be used to determine the end-point of the reaction of a strong acid and a strong base
Strong acids with weak bases

- Lower starting pH compared to the previous experiment
- Sharp change in pH over a range (eg: pH 3.5 to pH 7.5)
- Only indicators with pH range within this range can be used
Weak acids with strong bases

- Sharp change in pH over a range (eg: pH 7.5 to pH 11)
Weak acids with weak bases

- No sharp change in pH over a large range
- No acid-base indicator is suitable to determine the end-point of this reaction
- Only a gradual colour change on addition of acid
