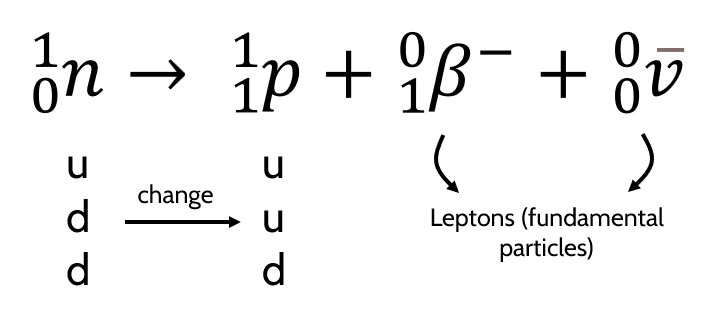AS Level Physics 9702
11. Particle physics
Written by: Adhulan Rajkamal
Formatted by: Adhulan Rajkamal
Index
11.1 Atoms, nuclei and radiation
- Atoms of all elements are made up of 3 subatomic particles → protons, neutrons and electrons
- Proton number (\(Z\)) → Number of protons
- Nucleon number (\(A\)) → Number of protons + neutrons
- A nucleon is a particle found in the nucleus of an atom → either a proton or a neutron
- Isotopes → Forms of the same element with different numbers of neutrons in their nuclei (same proton number but different nucleon number)
- Nuclide → A specific type of atom defined by its atomic number (\(Z\)) and mass number (\(A\))
- Nuclides are represented as follows:
$$ \begin{matrix} \text{nucleon number} \\ \text{proton number} \end{matrix} X = \begin{matrix} A \\ Z \end{matrix} X $$- \(X\) → Symbol of the element (the element is determined by the number of protons)
- In all nuclear processes (e.g., radioactive decay), the nucleon number and charge are conserved:
- Sum of all nucleon numbers before the nuclear process = Sum of all nucleon numbers after the nuclear process
- Sum of all charges before the nuclear process = Sum of all charges after the nuclear process
🔥Unified atomic mass unit (u)
- SI unit to measure mass of atoms and constituent particles is u (unified atomic mass unit) and not kg
| Proton | \(1u\) |
| Neutron | \(1u\) |
| Electron | \(\frac{1}{2000}u\) |
Radioactivity
- In some elements, the combination of protons and neutrons is such that the forces acting on them are unbalanced.
- Hence, to become more stable, they emit particles and/or electromagnetic (e.m.) waves → this is radioactivity.
- Common particles and e.m. waves emitted by radioactive nuclei:
- Alpha particle (\(\alpha\))
- Beta particle (\(\beta\))
- Gamma rays (\(\gamma\)) → e.m. radiation
Alpha Radiation
- Composition → 2 protons + 2 neutrons (Helium nucleus)
- Mass → \(4u\)
- Charge → \(+2\)
- Range in air → few cm
- Denoted as → \(\,^{4}_{2}\alpha\)
- Example equation:
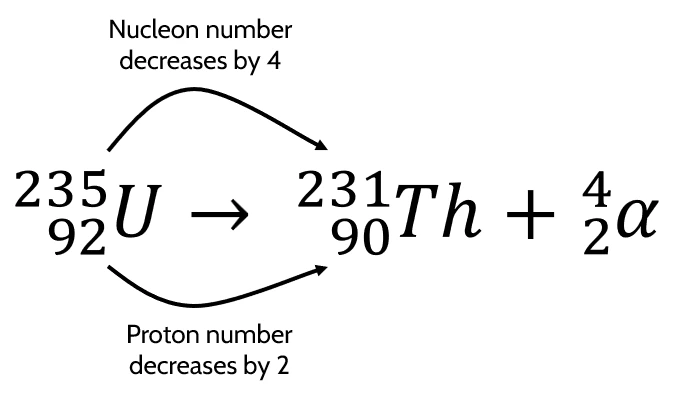
-
- Total nucleon number after nuclear process = \(231 + 4 = 235\)
- Total charge after nuclear process = \(90 + 2 = 92\)
Beta Radiation
- Beta radiation consists of two types:
- Beta minus (\(\beta^-\)) → emission of electrons
- Mass → \(\frac{1}{2000}u\) (can be considered zero)
- Charge → \(-1\)
- Beta plus (\(\beta^+\)) → emission of positrons
- Mass → \(\frac{1}{2000}u\) (can be considered zero)
- Charge → \(+1\) (opposite of \(\beta^-\))
- Beta minus (\(\beta^-\)) → emission of electrons
🔥 Anti-particle
- An antiparticle has the same mass but the opposite charge to the corresponding particle.
- Positrons (\(\beta^+\)) are the antiparticles of electrons (\(\beta^-\)).
- Example equation for \(\beta^-\) decay:
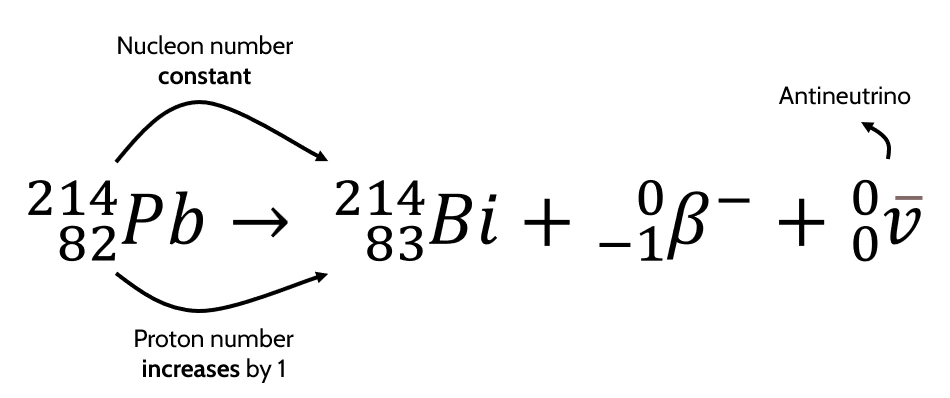
-
-
Antineutrino → additional particle emitted during \(\beta^-\) decay
- The dash above denotes that it is an antiparticle (antineutrinos are the antiparticles of the neutrino).
- It has 0 mass and 0 charge.
- Total nucleon number after nuclear process = \(214 + 0 + 0 = 214\)
- Total charge after nuclear process = \(83 – 1 + 0 = 82\)
-
Antineutrino → additional particle emitted during \(\beta^-\) decay
- Changes in the nucleus during \(\beta^-\) decay:
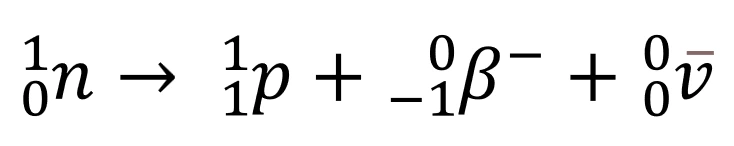
-
- 1 neutron changes to a proton, \(\beta^-\) (electron), and an antineutrino.
- Note that the charge and nucleon number are conserved in the above equation.
- Tip: Use this equation to explain the changes in nucleon and proton numbers in the example equation for \(\beta^-\) decay.
- Example equation for \(\beta^+\) decay:
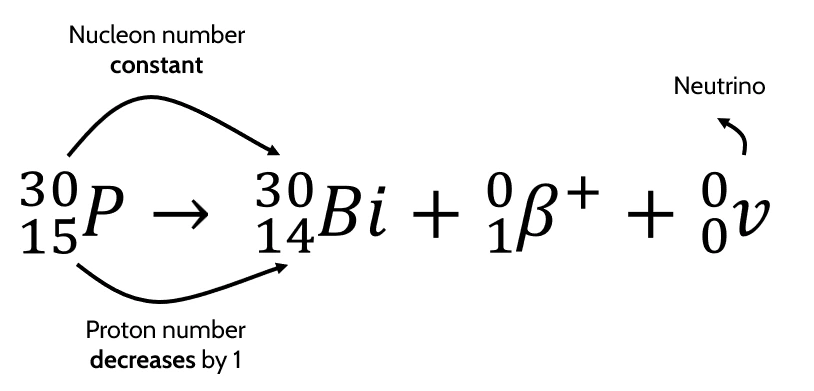
-
-
Neutrino → additional particle emitted during \(\beta^+\) decay (antineutrino, emitted in \(\beta^-\) decay, is its antiparticle)
- Neutrino has 0 mass and 0 charge.
- Total nucleon number after nuclear process = \(30 + 0 + 0 = 30\)
- Total charge after nuclear process = \(14 + 1 + 0 = 15\)
-
Neutrino → additional particle emitted during \(\beta^+\) decay (antineutrino, emitted in \(\beta^-\) decay, is its antiparticle)
- Changes in the nucleus during \(\beta^+\) decay:
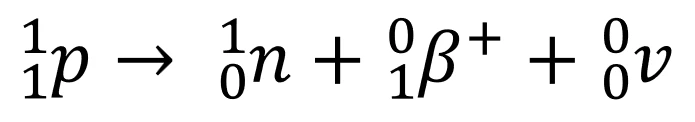
-
- 1 proton changes to neutron, \(\beta^+\) (positron) and neutrino (opposite of \(\beta^-\))
- Note that the charge and nucleon number is conserved in the above equation.
- Tip: Use this equation to explain the changes in nucleon and proton numbers in the example equation for \(\beta^+\) decay.
Gamma radiation
- Composition → gamma rays (electromagnetic radiation)
- Mass → 0
- Charge → 0 (neutral)
- Example equation:
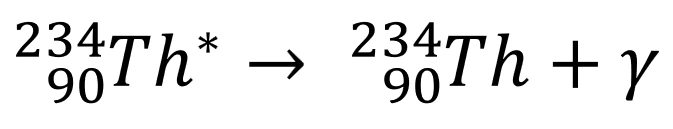
-
- \(*\) represents “excited state” → i.e. the Thorium atom is unstable
- No change in the composition of the nucleus
- Note that nucleon number and charge are conserved
| Radiation type | Composition | Mass | Charge |
|---|---|---|---|
| \(\alpha\) (Alpha) | Helium nucleus (2 protons + 2 neutrons) | \( 4u \) | +2 |
| \(\beta^-\) (Beta minus) | Electron | \( \frac{1}{2000}u \) (negligible) | -1 |
| \(\beta^+\) (Beta plus) | Positron (anti-electron) | \( \frac{1}{2000}u \) (negligible) | +1 |
| \(\gamma\) (Gamma) | Electromagnetic wave | 0 | 0 (neutral) |
Kinetic energy of emitted alpha and beta particles
- The \(\alpha\)-particles emitted from a particular radioactive nuclide all have the same kinetic energy
- The \(\beta\)-particles emitted from a particular radioactive nuclide have a continuous range of kinetic energies because neutrinos or antineutrinos are emitted
- The total energy is shared between the beta particle and neutrino/antineutrino
Alpha particle scattering experiment
- Setup:
- Alpha particles (positively charged helium nuclei) were directed at a thin gold foil
- Detectors measured the scattering pattern
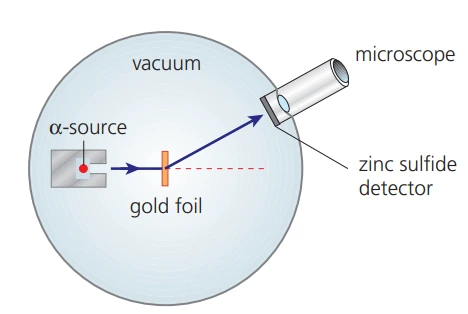
- Observations:
- Most \(\alpha\)-particles passed straight through the foil: indicates atoms are mostly empty space
- A few \(\alpha\)-particles deflected at small angles: suggests a repulsive force, implying a positive charge concentrated in the atom
- Very few \(\alpha\)-particles deflected at large angles (even backward): demonstrates the existence of a small, dense, positively charged nucleus
11.2 Fundamental particles
- There are two types of fundamental particles
- Quarks
- Leptons
- There are 6 flavours/types of quarks:
| Flavour of quark | Charge |
|---|---|
| Up (u) | \( + \frac{2}{3} e \) (positive of the two-thirds of the charge of an electron) |
| Top (t) | |
| Charm (c) | |
| Down (d) | \( – \frac{1}{3} e \) (one-third of the charge of an electron) |
| Bottom (b) | |
| Strange (s) |
- The antiparticles of the quarks have the same mass as the quark but the opposite charge
- Eg: Anti-up quark (\(\bar{u}\)) has the same mass as an up quark but a charge of \( -\frac{2}{3}e \)
- Electrons and neutrinos (and their antiparticles) are all leptons
- Subatomic particles (protons, neutrons, and electrons) are placed into two main categories:
| Protons and neutrons (those affected by strong forces) | Electrons (those not affected by strong forces) |
| Not fundamental particles – hadrons are made up of quarks (a fundamental particle) | Considered as a fundamental particle |
- There are two types of hadrons:
| Made of 3 quarks | Made of one quark and one anti-quark |
| Eg: Protons and neutrons | Eg: Pions and kappas |
- Quark composition of a proton:
- Protons are made of 3 up quarks
- The composition is denoted as → \( u, u, u \)
- Charge of a proton:
- \( \frac{2}{3}e + \frac{2}{3}e + \frac{2}{3}e = +1e \)
- Quark composition of a neutron:
- Neutrons are made of 1 up quark and 2 down quarks
- The composition is denoted as → \( u, d, d \)
- Charge of a neutron:
- \( \frac{2}{3}e – \frac{1}{3}e – \frac{1}{3}e = 0 \)
Changes in quark formation – Beta decay
- Changes in quark formation during \( \beta^- \) decay: