AS Level Chemistry 9701
9. The Periodic Table: chemical periodicity
Written by: Adhulan Rajkamal
Formatted by: Pranav I
Index
✅ Periodicity (definition)
The repeating trends in physical and chemical properties of elements across periods
9.1 Periodicity of physical properties
- Atomic radius decreases across a period
- Nuclear charge increases – pulls the electrons in the outer shell closer
- The same number of shells → shielding effect constant
Periodic properties of ionic radii
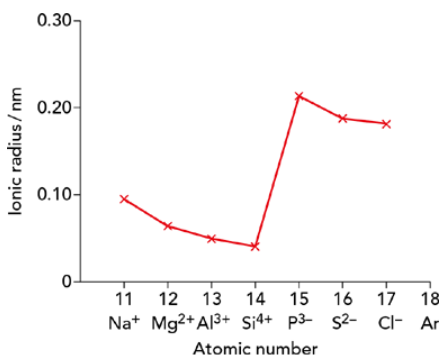
- Cations
- Cations are much smaller than their parent atoms
- They lose their outermost electron shell
- There is less electron shielding compared to the parent atom
- As the nuclear charge increases across the period
- The valence electrons are pulled closer to the nucleus, reducing the ionic radius
- Cations are much smaller than their parent atoms
- Anions
- Anions are larger than their parent atoms
- Gaining extra electrons increases electron-electron repulsion in the valence shell, expanding the ionic radius
- Across the period, ionic radii of anions decrease
- The increase in nuclear charge pulls electrons closer, reducing the size despite repulsion
- Anions are larger than their parent atoms
Periodic patterns of melting points and electrical conductivity
- To explain the trend in m.p. and electrical conductivity we will consider the bonding and structure of elements

Melting point
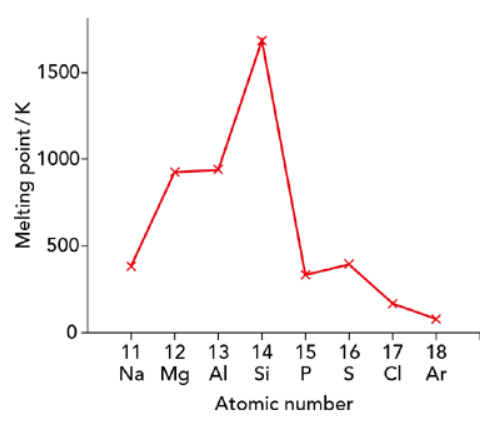
- Increases from Na to Al: positive ion charge increases (+1 to +3) and more delocalized electrons → stronger metallic bonds
- Sharp rise at Si: giant covalent structure → high energy needed to break covalent bonds
- Sharp drop after Si: simple molecular structures with weak intermolecular (id-id) forces → lower m.p. than Na
- Small rise at S: S₈ molecules have more electrons than P₄ → stronger id-id forces
- Decreases from S to Ar: fewer electrons per molecule → weaker id-id forces → lower m.p

Electrical conductivity

- Increases from Na to Al: positive ion charge increases (+1 to +3) and more delocalized electrons → stronger metallic bonds
- Sharp rise at Si: giant covalent structure → high energy needed to break covalent bonds
- Sharp drop after Si: simple molecular structures with weak intermolecular (id-id) forces → lower m.p. than Na
- Small rise at S: S₈ molecules have more electrons than P₄ → stronger id-id forces
- Decreases from S to Ar: fewer electrons per molecule → weaker id-id forces → lower m.p
9.2 Periodicity of chemical properties
Reactions of Period 3 elements with oxygen
| # | Element | Observation | Equation | Structure of Oxide |
|---|---|---|---|---|
| 1 | Na | Yellow flame; white oxide | 4Na(s) + O2(g) → 2Na2O(s) | Giant ionic |
| 2 | Mg | Bright white flame; white oxide | 2Mg(s) + O2(g) → 2MgO(s) | Giant ionic |
| 3 | Al | White oxide | 4Al(s) + 3O2(g) → 2Al2O3(s) | Giant ionic |
| 4 | Si | Burns if heated strongly | Si(s) + O2(g) → SiO2(g) | Giant covalent |
| 5 | P | Yellow/white flame; clouds of white smoke | P4(s) + 3O2(g) → P4O6(s) P4(s) + 5O2(g) → P4O10(s) | Simple covalent |
| 6 | S | Blue flame; colorless gas | S(s) + O2(g) → SO2(g) 2SO2(g) + O2(g) → 2SO3(g) | Simple covalent |
| 7 | Cl | Does not react directly with O2 | Cl2O7 or Cl2O | Not stated |
Reactions of Period 3 oxides
| # | Period 3 Element | Oxide in Water | Equation (Oxide + Water) | Nature of Oxide | Equation (Acid-Base Characteristics) | Oxidation State |
|---|---|---|---|---|---|---|
| 1 | Na | Dissolves exothermically (pH = 14) | Na₂O + H₂O → 2NaOH | Alkaline | Na₂O + 2HCl → 2NaCl + H₂O | +1 |
| 2 | Mg | Slightly soluble (pH = 9) | MgO + H₂O → Mg(OH)₂ | Basic | MgO + 2HCl → MgCl₂ + H₂O | +2 |
| 3 | Al | Insoluble | N/A | Amphoteric | Al₂O₃ + 6HCl → 2AlCl₃ + 3H₂O Al₂O₃ + 2NaOH → 2NaAlO₂ + H₂O | +3 |
| 4 | Si | Insoluble | N/A | Acidic | SiO₂ + CaO → CaSiO₃ SiO₂ + 2NaOH → Na₂SiO₃ + H₂O | +4 |
| 5 | P (P₄O₆) | Reacts with cold water (pH = 1–2) | P₄O₆ + 6H₂O → 4H₃PO₃ | Acidic | P₄O₆ + 12NaOH → 4Na₃PO₃ + 6H₂O | +3 |
| P (P₄O₁₀) | Reacts violently with water (pH = 1–2) | P₄O₁₀ + 6H₂O → 4H₃PO₄ | P₄O₁₀ + 12NaOH → 4Na₃PO₄ + 6H₂O | +5 | ||
| 6 | S (SO₂) | Dissolves readily (pH = 1) | SO₂ + H₂O → H₂SO₃ | Acidic | SO₂ + 2NaOH → Na₂SO₃ + H₂O SO₂ + CaO → CaSO₃ | +4 |
| S (SO₃) | Reacts violently (pH = 0) | SO₃ + H₂O → H₂SO₄ | SO₃ + 2NaOH → Na₂SO₄ + H₂O SO₃ + CaO → CaSO₄ | +6 | ||
| 7 | Cl | +1, +6, +7 | ||||
🚨 The maximum oxidation state for elements in compounds depends on the number of electrons in their outermost principal quantum shell
Reactions of Period 3 elements with chlorine
| # | Element | Equation | Bonding and Structure |
|---|---|---|---|
| 1 | Na | 2Na(s) + Cl₂(g) → 2NaCl(s) | Ionic bonding; giant ionic |
| 2 | Mg | Mg(s) + Cl₂(g) → MgCl₂(s) | |
| 3 | Al |
2Al(s) + 3Cl₂(g) → 2AlCl₃(s) 2Al(s) + 3Cl₂(g) → Al₂Cl₆(g) | Covalent bond; simple molecular |
| 4 | Si | Si(s) + 2Cl₂(g) → SiCl₄(l) (colourless liquid) | |
| 5 | P |
P₄(s) + 6Cl₂(g) → 4PCl₃(l) (fuming, colourless liquid) P₄(s) + 10Cl₂(g) → 4PCl₅(s) (off-white solid) |
Reactions of Period 3 chlorides with water
| # | Element | Chloride in Water | Equation |
|---|---|---|---|
| 1 | Na | Dissolves (pH = 7) | NaCl(s) + aq → Na⁺(aq) + Cl⁻(aq) |
| 2 | Mg | Dissolves with slight hydrolysis (pH = 6.5) | MgCl₂(s) + aq → Mg²⁺(aq) + 2Cl⁻(aq) |
| 3 | Al | Hydrolysis (pH = 3) | AlCl₃(s) + 6H₂O(l) → [Al(H₂O)₆]³⁺ + 3Cl⁻ (exothermic) |
| 4 | Si | Hydrolysis (pH = 2) | SiCl₄(l) + 2H₂O(l) → SiO₂(s) + 4HCl(g) (steamy fumes of HCl) |
| 5 | P | Violent hydrolysis (pH = 2) | PCl₃(l) + 3H₂O(l) → H₃PO₃(aq) + 3HCl(g) (steamy fumes of HCl) PCl₅(s) + 4H₂O(l) → H₃PO₄(aq) + 5HCl(g) |
Reactions of Period 3 elements with water
| # | Element | Observation | Equation |
|---|---|---|---|
| 1 | Na |
– Catches fire in cold H₂O – Violent exothermic reaction | 2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g) |
| 2 | Mg | Slow reaction | Mg(s) + 2H₂O(l) → Mg(OH)₂(aq) + H₂(g) |
| Rapid reaction | MgO + H₂(g) |
