AS Level Chemistry 9701
19. Nitrogen compounds
Written by: Pranav I
Formatted by: Pranav I
Index
19.1 Primary amines
- Reagent: NH3
- Conditions: heat under pressure, and ethanol
- Primary amine formed
- If the ammonia is not in excess, a mixture of amines will be formed → primary amines will act as nucleophiles and attack haloalkanes to form secondary amines
\[
\text{C}_2\text{H}_5\text{Br} + \text{NH}_3 \rightarrow \text{C}_2\text{H}_5\text{NH}_2 + \text{HBr} \, (\textit{excess } \text{NH}_3)
\]
\[
2\text{C}_2\text{H}_5\text{Br} + \text{NH}_3 \rightarrow (\text{C}_2\text{H}_5)_2\text{NH} + 2\text{HBr} \, (\textit{without excess } \text{NH}_3)
\]
19.2 Nitriles and hydroxynitriles
Substitution with cyanide ions, CN- (in ethanol)
- Reagent: NaCN
- Conditions: heat under reflux, and ethanol
- Adds an extra carbon atom to the compound (original haloalkane carbon chain)
\[
\text{C}_2\text{H}_5\text{Br} + \text{CN}^- \rightarrow \text{C}_2\text{H}_5\text{CN} + \text{Br}^-
\]
Nucleophilic addition with HCN
- Addition takes place across the C=O bond → attacked by a nucleophile
- HCN is generated in situ by the reaction of KCN and dilute H2SO4
- Nitrile group added → increase in the number of C in the original carbonyl compound
\[
\text{CH}_3\text{CH}_2\text{CHO} + \text{HCN} \rightarrow{\text{CN}^-} \text{CH}_3\text{CH}_2\text{CH(OH)CN}
\]
Mechanism of nucleophilic addition
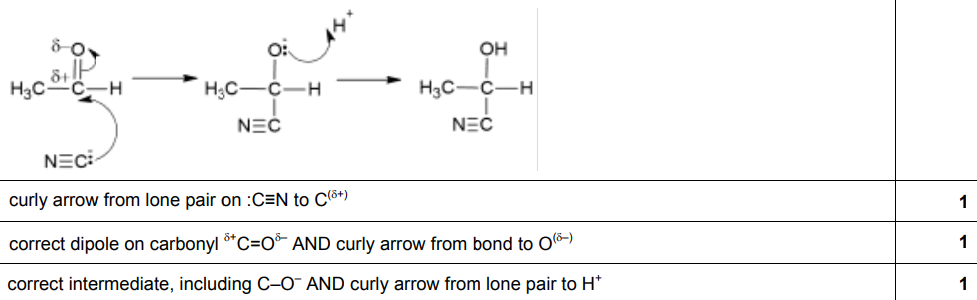
- >C=O is polarised → high electronegativity of the oxygen atom
- C has δ+ and O has δ-
- Open to attack by a nucleophile
- Step 1
- CN– nucleophile attacks the δ+ C atom
- One bond in C=O breaks heterolytically
- O gains a negative charge
- Step 2
- Lone pair of electrons from O– attacks H+
- This forms a hydroxy group
Hydrolysis of nitrles
- Nitrile group (-C≡N) can be hydrolyzed to a carboxylic acid
- Reagent: HCl
- Conditions: dilute & heat under reflux
\[
\text{CH}_3\text{CH}_2\text{CN} + 2\text{H}_2\text{O} + \text{HCl} \rightarrow \text{CH}_3\text{CH}_2\text{COOH} + \text{NH}_4\text{Cl}
\]
- Reagent: alkali (e.g. NaOH)
- Conditions: dilute & heat under reflux
- It must be acidified to produce a carboxylic acid
\[
\text{CH}_3\text{CH}_2\text{CN} + 2\text{H}_2\text{O} + \text{NaOH} \rightarrow \text{CH}_3\text{CH}_2\text{COONa} + \text{NH}_3 \quad \text{(Alkaline hydrolysis)}
\]
\[
\text{CH}_3\text{CH}_2\text{COONa} + \text{HCl} \rightarrow \text{CH}_3\text{CH}_2\text{COOH} + \text{NaCl} \quad \text{(Acidification)}
\]
