AS Level Chemistry 9701
15. Halogen compounds
Written by: Pranav I
Formatted by: Pranav I
Index
15.1 Making haloalkanes
| # | Reagent | Acts on | Reaction type | Conditions | Example |
|---|---|---|---|---|---|
| 1 | Cl2 | Alkanes | Free Radical Substitution | UV light | \[ \text{CH}_4 + \text{Cl}_2 \rightarrow{\text{UV light}} \text{CH}_3\text{Cl} + \text{HCl} \] |
| 2 | Br2 | Alkenes | Electrophilic Addition | Room temperature | \[ \text{CH}_2\text{=CH}_2 + \text{Br}_2 \rightarrow \text{CH}_2\text{Br-CH}_2\text{Br} \] |
| 3 | HCl | Alkenes | Electrophilic Addition | Room temperature | \[ \text{CH}_2\text{=CH}_2 + \text{HCl} \rightarrow \text{CH}_3\text{CH}_2\text{Cl} \] |
| 4 | SOCl2 | Alcohols | Substitution | Room temperature | \[ \text{CH}_3\text{CH}_2\text{OH} + \text{SOCl}_2 \rightarrow \text{CH}_3\text{CH}_2\text{Cl} + \text{SO}_2 + \text{HCl} \] |
| 5 | PCl5 | Alcohols | Substitution | Room temperature | \[ \text{CH}_3\text{CH}_2\text{OH} + \text{PCl}_5 \rightarrow \text{CH}_3\text{CH}_2\text{Cl} + \text{POCl}_3 + \text{HCl} \] |
| 6 | PCl3 | Alcohols | Substitution | Heat | \[ 3\text{CH}_3\text{CH}_2\text{OH} + \text{PCl}_3 \rightarrow 3\text{CH}_3\text{CH}_2\text{Cl} + \text{H}_3\text{PO}_3 \] |
- Primary haloalkanes
- X-bonded C bonded to one alkyl group
- Example: C2H5Cl
- Secondary haloalkanes
- X-bonded C bonded to two alkyl groups
- Example: (CH3)2CHCl
- Tertiary haloalkanes
- X-bonded C bonded to three alkyl groups
- Example: (CH3)3Cl
15.2 Nucleophilic substitution reactions
- Nucleophilic substitution: the mechanism of the organic reaction in which a nucleophile attacks a carbon atom carrying a partial positive charge, resulting in the replacement of an atom carrying a partial negative charge by the nucleophile
- Haloalkanes are reactive due to the polar nature of the C-X bond (between a carbon and a halogen)
- Carbon → partial positive charge (δ+)
- Halogen → partial negative charge (δ–)
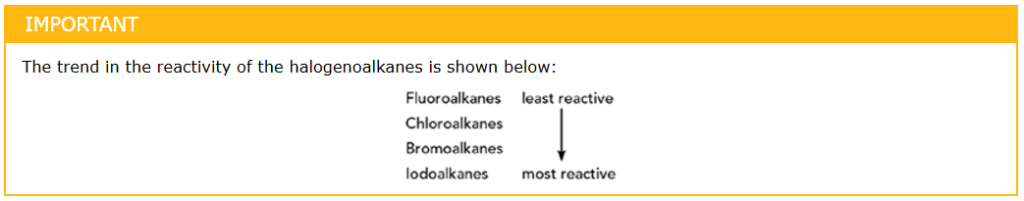
- C-F bond is the strongest
- C-I bond is the weakest → broken easily during reactions
Substitution reactions with aqueous alkali, OH-(aq)
- Reagent: NaOH (or a different alkali)
- Behaves as a nucleophile → donates a pair of electrons to the C with a δ+ charge
- Conditions: heat, aqueous
- The halogen atom in the haloalkane is replaced by a hydroxyl (OH–) group, forming an alcohol
- This is also a hydrolysis reaction
\[
\text{C}_2\text{H}_5\text{Br} + \text{OH}^- \rightarrow \text{C}_2\text{H}_5\text{OH} + \text{Br}^-
\]
Substitution with cyanide ions, CN- (in ethanol)
- Reagent: NaCN
- Conditions: heat under reflux, and ethanol
- Adds an extra carbon atom to the compound (original haloalkane carbon chain)
\[
\text{C}_2\text{H}_5\text{Br} + \text{CN}^- \rightarrow \text{C}_2\text{H}_5\text{CN} + \text{Br}^-
\]
Substitution with ammonia, NH3 (in ethanol)
- Reagent: NH3
- Conditions: heat under pressure, and ethanol
- Primary amine formed
- If the ammonia is not in excess, a mixture of amines will be formed → primary amines will act as nucleophiles and attack haloalkanes to form secondary amines
\[
\text{C}_2\text{H}_5\text{Br} + \text{NH}_3 \rightarrow \text{C}_2\text{H}_5\text{NH}_2 + \text{HBr} \, (\textit{excess } \text{NH}_3)
\]
\[
2\text{C}_2\text{H}_5\text{Br} + \text{NH}_3 \rightarrow (\text{C}_2\text{H}_5)_2\text{NH} + 2\text{HBr} \, (\textit{without excess } \text{NH}_3)
\]
15.3 Mechanism of nucleophilic substitution of haloalkanes
- Carbon-halogen bond is polarized → halogen is more electronegative than carbon
- Nucleophile attacks the carbon atom (δ+) bonded to the halogen
- Nucleophiles are electron pair donors which are attracted to electron-deficient atoms
- Halogen atom is replaced by the nucleophile
Mechanism for primary halogenoalkanes (SN2)
- The nucleophile donates a pair of electrons to the δ+ carbon atom → forms a new covalent bond
- C-X bond breaks at the same time
- X atom takes both the electrons (heterolytic fission) → X– ion formed
- S – Substitution
- N – Nucleophilic
- 2 – the reaction rate (determined by the slow step in the mechanism) involves two reacting species
- Dependent on the concentrations of the haloalkanes and the hydroxide ions

Mechanism for tertiary halogenoalkanes (SN1)
- The reaction involves a two-step mechanism
- Step 1 → breaking of the carbon-halogen bond
- Forms a tertiary carbocation
- Br– forms by heterolytic fission
- Step 2 → tertiary carbocation is attacked by the nucleophile
- ‘1’ in SN1 – reaction rate depends on one reagent only (concentration of the haloalkane → in the slow step)

- Both the SN1 and SN2 mechanisms are likely to be a part of the nucleophilic substitution of secondary halogenoalkanes
15.4 Elimination reactions
- It involves the loss of a small molecule from the original organic molecule
- Hydrogen halide (e.g. HCl; HBr) in this case
- Reagent: NaOH
- Conditions: heat, and ethanol
- Ethanolic OH– ion acts as a base → accepts H+ ion from the haloalkane to form water and a salt
- Note: The C-X bond breaks heterolytically → forms a Br– ion and produces an alkene
\[
\text{C}_2\text{H}_5\text{Br} + \text{NaOH(ethanol)} \rightarrow \text{CH}_2=\text{CH}_2 + \text{NaBr} + \text{H}_2\text{O}
\]
