AS Level Biology 9700
2. Biological molecules
Written by: Rhia Sakthivel
Formatted by: Pranav I
Index
2.1 Biochemistry
- Biochemistry studies the chemical reactions of biological molecules
- Molecular structure is closely linked to biological functions
- Metabolism: total of all biochemical reactions in the body.
2.2 The building blocks of life
- The four most common elements in living organisms are hydrogen, carbon, oxygen, and nitrogen
- These elements make up over 99% of the atoms in living things
- Carbon is crucial due to its ability to form long chains or ring structures
- Carbon skeletons serve as the foundation of organic molecules
- Before life evolved, chemical evolution led to the formation of simple carbon-based biological molecules
- Simple biological molecules are limited in variety and act as building blocks for larger, complex molecules
2.3 Monomers, polymers and macromolecules
Macromolecules
- Macromolecules are giant molecules in living organisms, categorized into:
- Polysaccharides
- Proteins (polypeptides)
- Nucleic acids (polynucleotides)
- The term “poly” means “many,” as macromolecules (polymers) consist of many repeating subunits (monomers)
Monomer
✅ Monomer (definition)
Simple molecules used as a basic building block for polymers
- Examples: monosaccharides, amino acids, nucleotides
- Monomers are connected by covalent bonds, which are strong bonds formed by sharing electrons:
- Examples include glycosidic, ester, and peptide bonds
Polymer
✅ Polymer (definition)
Giant molecules consisting of many repeating subunits (monomers); joined together by covalent bonds; through a condensation reaction.
- Forming polymers involves condensation reactions, where two monomers join by removing a water molecule
- Breaking down polymers requires hydrolysis, where water is added to split the bonds
- Monomers and their corresponding polymers:
- Monosaccharides → polysaccharides
- Amino acids → proteins
- Nucleotides → nucleic acids
2.4 Carbohydrates
- Carbohydrates are composed of carbon, hydrogen, and oxygen atoms
- Hydrogen and oxygen are present in a 2:1 ratio
- General formula: Cx(H2O)y
- Carbohydrates are classified into three groups:
- Monosaccharides
- Disaccharides
- Polysaccharides (not sugars)
Monosaccharides
- Monomers of polysaccharides
- E.g. glucose, fructose, galactose
- Joined together by glycosidic bonds during condensation
- Monosaccharides are sugars that dissolve easily in water to form sweet solutions
- Composed of a single sugar molecule (“mono” = one, “saccharide” = sugar)
- General formula: (CH₂O)ₙ
- Classified by the number of carbon atoms:
- Trioses (3C)
- Pentoses (5C)
- E.g. ribose, deoxyribose
- Hexoses (6C)
- E.g. glucose, fructose, galactose
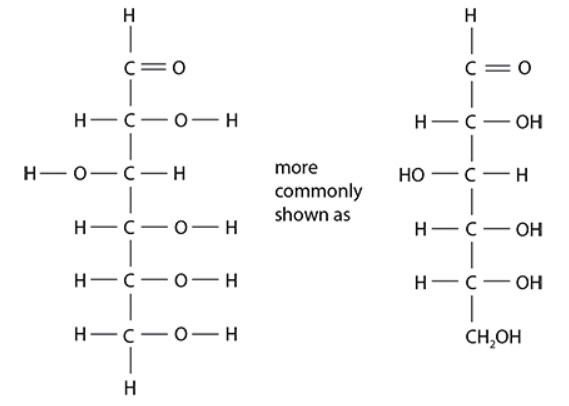
- Molecular formula for hexoses: C₆H₁₂O₆
- The structural formula shows the arrangement of atoms
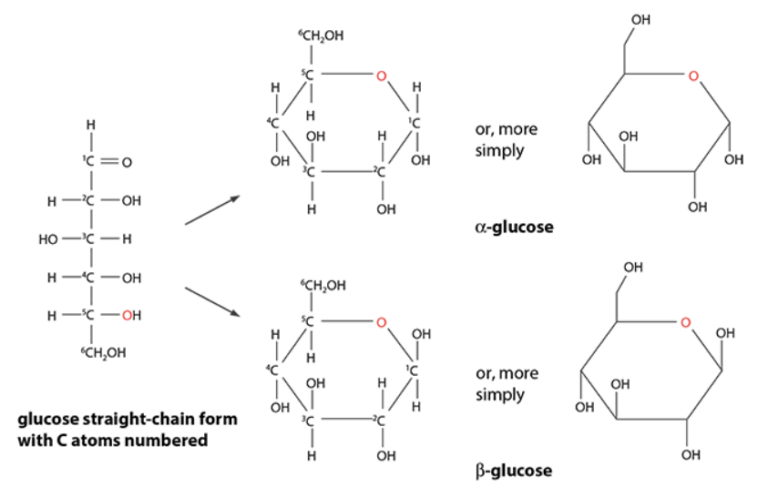
- Ring structures:
- Pentoses and hexoses can form stable rings
- In glucose, carbon 1 joins oxygen on carbon 5
- α-glucose → OH on carbon 1 is below the ring
- β-glucose→ OH on carbon 1 is above the ring
- These are isomers (different forms of the same chemical)
- Functions of monosaccharides
- Energy source in respiration
- Many carbon-hydrogen bonds release energy when broken
- Energy helps make ATP from ADP and phosphate
- Glucose is vital for energy metabolism
- Building blocks for larger molecules:
- Glucose forms polysaccharides like starch, glycogen, and cellulose
- Ribose forms RNA and ATP
- Deoxyribose forms DNA
- Energy source in respiration
Disaccharides
✅ Disaccharide (definition)
A simple sugar molecule consisting of two monosaccharides joined together by glycosidic bonds through a condensation reaction
- Disaccharides are sugars formed by joining two monosaccharides (“di” = two)
- Common disaccharides
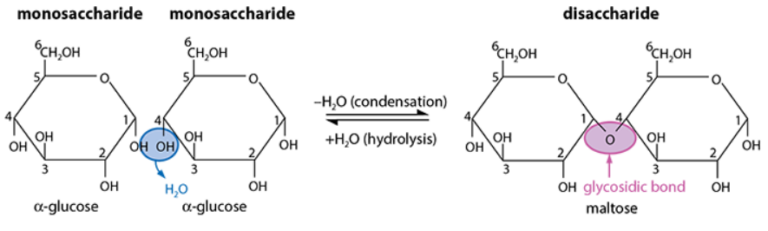
- Maltose: glucose + glucose
- Sucrose: glucose + fructose (transport sugar in plants; table sugar)
- Lactose: glucose + galactose (milk sugar, essential for young mammals)
- Formation
- Condensation reaction
- Two –OH groups align
- One –OH combines with hydrogen from another to form water
- An oxygen bridge forms, creating a glycosidic bond between the monosaccharides
- Condensation reaction
- Breaking down
- Hydrolysis reaction
- Water is added to break the glycosidic bond
- It occurs during digestion to convert disaccharides into monosaccharides
- Hydrolysis reaction
- Many possible disaccharides exist due to multiple –OH groups in monosaccharides
- Enzymes control which –OH groups align, so only a few disaccharides are common in nature
Polysaccharides
✅ Polysaccharide (definition)
Polymers of many repeating units of monosaccharides are joined together by many glycosidic bonds
- Composition: can be several thousand monosaccharide units long, forming macromolecules
- Examples: starch, glycogen, and cellulose
- Polysaccharides are not sugars
Starch and glycogen
- Purpose: store glucose in a compact, inert, insoluble form
- To maintain osmotic balance
- To avoid glucose reactivity interfering with cell chemistry
- In plants → starch
- In animals → glycogen
- Starch
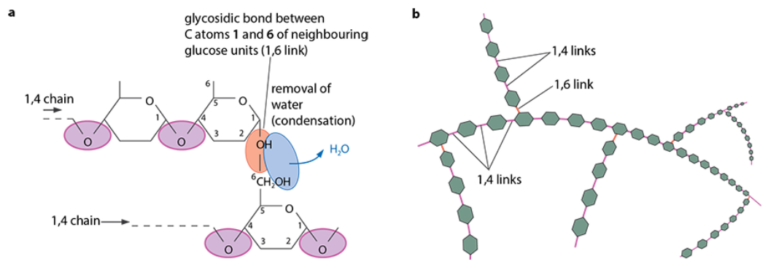
- A mixture of two substances → amylose and amylopectin
- Amylose: long, unbranched chains of α-glucose (1,4 linkages), forming a helical, compact structure
- Amylopectin: branched chains with 1,4 and 1,6 linkages
- Starch forms grains found in plant storage organs like potato tubers and cereal seeds
- A mixture of two substances → amylose and amylopectin
- Glycogen:
- Similar to amylopectin but more branched
- Found in liver and muscle cells as an energy reserve
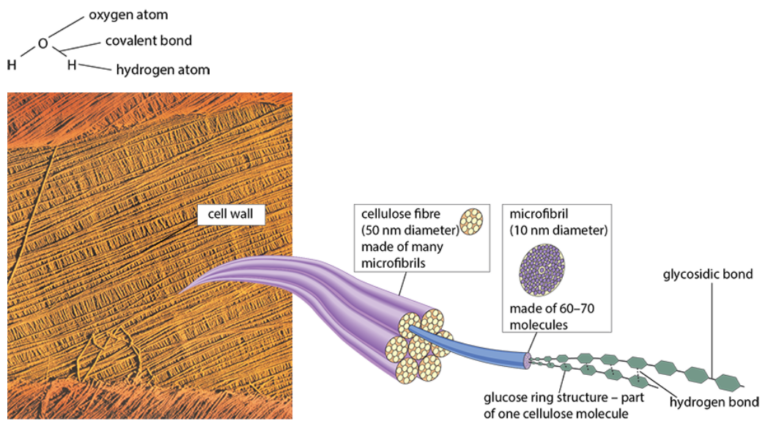
Cellulose
- Most abundant organic molecule due to presence in plant cell walls
- Function: structural role due to high mechanical strength
- Composition: polymer of β-glucose
- Formation
- Glycosidic bonds between carbon 1 of one β-glucose and carbon 4 of another, with alternating glucose molecules rotated 180°
- Hydrogen bonds between –OH groups within and between molecules provide strength
- Structure
- Molecules form microfibrils (bundles of 60–70 molecules), which further bundle into fibers
- Fibers arranged in layers for tensile strength and flexibility
- Formation
- Properties
- High tensile strength (withstands osmotic pressure)
- Freely permeable, allowing water and solutes to pass
Dipoles and hydrogen bonds
- Dipole formation: unequal electron sharing creates partial charges (δ+ on hydrogen, δ− on oxygen)
- Hydrogen bonds
- Weak but significant forces between molecules with dipoles (e.g., –OH, –CO, –NH groups)
- Affect structure and properties of carbohydrates and proteins
- Polarity and solubility
- Polar molecules (e.g. sugars) are hydrophilic (water-soluble)
- Non-polar molecules are hydrophobic (insoluble in water)
2.5 Lipids
- A diverse group of organic molecules that are insoluble in water
- Formation: most lipids result from fatty acids combining with an alcohol
- Types
- Fats: solid at room temperature
- Oils: liquid at room temperature
- Similarity: fats and oils are chemically very similar despite physical differences
Fatty acids
- Structure: consist of a carboxyl group (–COOH) at one end and a hydrocarbon tail (usually 15 or 17 carbon atoms long)
- Saturation
- Unsaturated → fatty acids with double bonds between carbon atoms (e.g. oils like olive oil)
- Monounsaturated → one double bond
- Polyunsaturated → multiple double bonds
- Saturated → fatty acids without double bonds, typically found in animal fats
Alcohols and esters
- Alcohols: organic molecules containing a hydroxyl group (–OH)
- Glycerol: a three-hydroxyl alcohol
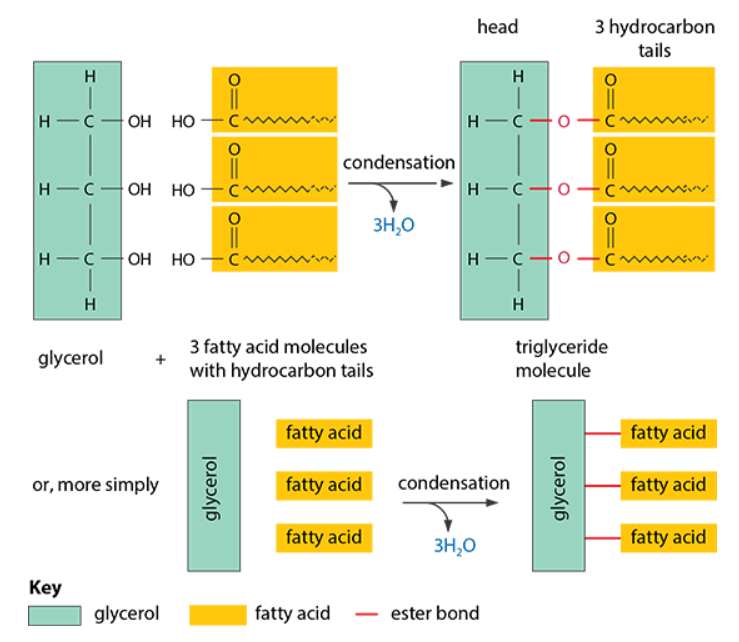
- Ester formation: when fatty acids react with glycerol, they form ester bonds and produce esters, like triglycerides, through condensation (water is released)
Triglycerides
- Structure: made from glycerol and three fatty acids, each connected via ester bonds
- The –COOH group on the acid reacts with the –OH group on the alcohol to form the ester bond, –COO–
- Properties
- Insoluble in water, soluble in organic solvents
- Hydrophobic (due to non-polar hydrocarbon tails)
- Functions
- Energy storage: rich in carbon-hydrogen bonds, yielding more energy than carbohydrates
- Insulation: stored beneath the skin and around organs for heat retention
- Metabolic water: provides water when oxidized in respiration (important for animals in dry habitats)
Phospholipids
- Structure: similar to triglycerides, but one fatty acid is replaced by a phosphate group, making one end hydrophilic (water-attracting) and the other hydrophobic (water-repelling)
- Function
- Form biological membranes, where hydrophilic heads face aqueous environments and hydrophobic tails form an impermeable barrier
- Important for cell membrane structure
2.6 Proteins
- Proteins make up more than 50% of the dry mass in most cells
- Functions of proteins include:
- All enzymes are proteins
- Essential components of cell membranes
- Some hormones are proteins (e.g. insulin, glucagon)
- Oxygen-carrying pigments like haemoglobin and myoglobin
- Antibodies that attack microorganisms
- Collagen provides strength to tissues (e.g. bones, arteries)
- Keratin found in hair, nails, and skin
- Actin and myosin are responsible for muscle contraction
- Storage proteins like casein in milk and ovalbumin in egg white
- All proteins are made from amino acids
Amino acids
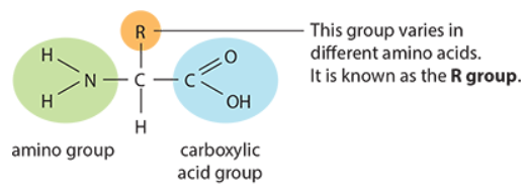
- Amino acids have a central carbon atom bonded to:
- Amino group → –NH2
- Carboxylic acid group → –COOH
- Hydrogen atom
- R group (fourth group) varies between amino acids, giving them unique properties
- There are 20 amino acids in proteins of living organisms, each with a different R group
- Amino acids have three-letter abbreviations
- Other amino acids have been synthetically created in laboratories
The peptide bond
- Peptide bond forms when:
- One amino acid loses a hydroxyl group (–OH) from its carboxylic acid group
- The other amino acid loses a hydrogen atom from its amino group
- The remaining carbon atom bonds with the nitrogen atom of the second amino acid
- Water (H2O) is released in the process (condensation reaction)
- Dipeptide: a molecule made of two linked amino acids
- Polypeptide: a chain of many amino acids linked by peptide bonds; can be one or more chains in a protein
- Protein synthesis occurs at ribosomes in living cells
- Protein breakdown involves hydrolysis (adding water) to break peptide bonds, releasing amino acids, which are absorbed into the blood
The primary structure
- Primary structure refers to the sequence of amino acids in a polypeptide chain
- This sequence determines the specific properties and function of the protein
- A change in just one amino acid can significantly alter the protein’s properties and function
- Polypeptides can have hundreds of amino acids linked in a specific sequence
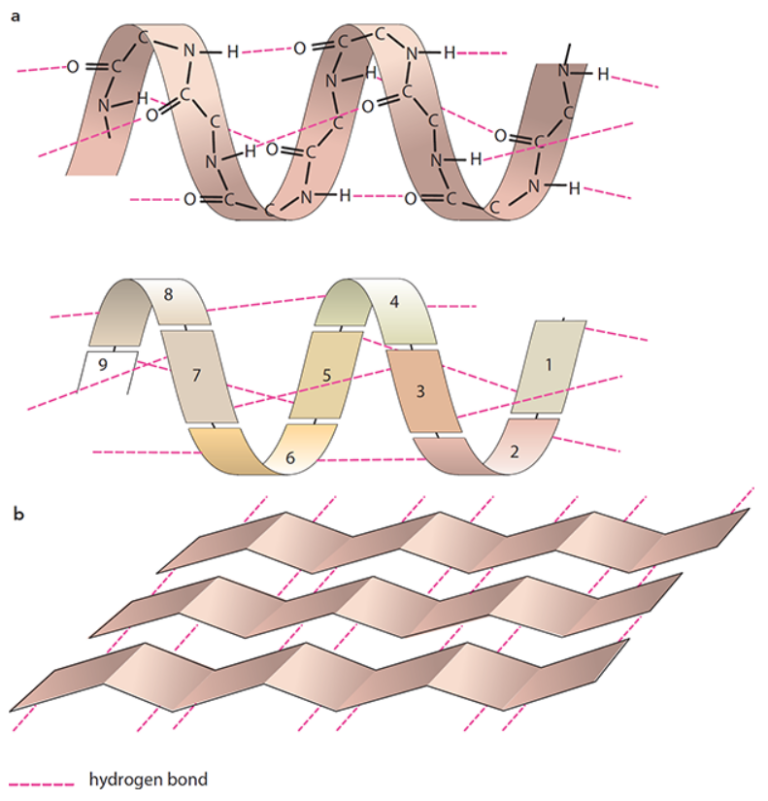
The secondary structure
- Secondary structure refers to the folding or coiling of a polypeptide chain due to interactions between amino acids
- An α-helix is a corkscrew shape stabilized by hydrogen bonds between the oxygen of one amino acid’s C=O group and the hydrogen of the –NH group of an amino acid four places ahead
- A β-pleated sheet is a straighter shape formed by hydrogen bonding between amino acids in parallel sheets, holding the structure in place
- Hydrogen bonds are strong but can be easily broken by high temperatures or pH changes
- Some proteins lack regular structures, depending on the interactions between R groups of amino acids
The tertiary structure
- Tertiary structure is the further folding of the secondary structure, forming a complex 3D shape
- The shape is held by bonds between amino acids in different parts of the chain
- Four types of bonds contribute to the stability of the tertiary structure
- Hydrogen bonds form between a variety of R groups; weak individually but strong in numbers
- Disulfide bonds form between 2 cysteine molecules (which contain sulfur atoms); the sulfur atoms create strong covalent bonds
- Ionic bonds form between R groups containing amino and carboxyl groups
- Hydrophobic interactions occur between non-polar R groups,
- Non-polar R groups are hydrophobic and avoid water
- In watery environments, the hydrophobic R groups come together (hydrophilic R groups outwards), forming the shape
The quarternary structure
- Quaternary structure refers to the overall structure formed by multiple polypeptide chains
- Example: haemoglobin (which has four polypeptide chains)
- The polypeptide chains in quaternary structures are held together by the same 4 bonds as in the tertiary structure
Globular and fibrous proteins
- Globular proteins
- Curl into a ball shape (e.g. myoglobin, haemoglobin)
- Non-polar, hydrophobic R groups face the center; polar, hydrophilic R groups face the outside
- Typically soluble in water due to hydrophilic R groups
- Key role in metabolic reactions (e.g. enzymes)
- Fibrous proteins
- Form long strands
- Not usually soluble in water
- Primarily structural (e.g. keratin in hair, nails, and skin, collagen for structural support)
Haemoglobin - a globular protein
- Haemoglobin
- Oxygen-carrying pigment in red blood cells
- Globular protein with quaternary structure (four polypeptide chains)
- Composed of two α-globin (alpha-globin) and two β-globin (beta-globin) chains
- Nearly spherical shape, with hydrophobic R groups inside and hydrophilic R groups on the outside
- Interactions between hydrophobic R groups hold the structure; hydrophilic groups maintain solubility
- In sickle cell anaemia, a mutation replaces glutamic acid (polar) with valine (non-polar), reducing solubility and causing symptoms
- Haem group
- Each polypeptide chain of haemoglobin contains a haem group (prosthetic group)
- Haem group contains an iron atom that binds to one oxygen molecule (O2)
- One haemoglobin molecule can carry four oxygen molecules (eight oxygen atoms)
- Responsible for haemoglobin’s color
- Bright red when oxygenated (oxyhaemoglobin)
- Darker red when deoxygenated
Collagen - a fibrous protein
- Collagen
- The most common protein in animals, making up 25% of total protein in mammals
- Insoluble fibrous protein found in skin, tendons, cartilage, bones, teeth, and blood vessel walls
- Important structural protein in nearly all animals
- Collagen structure
- Composed of three polypeptide chains in a helical shape (not α-helix)
- Three helices form a triple helix or “rope” structure, held together by hydrogen and covalent bonds
- Every third amino acid is glycine, allowing the chains to lie close together and form a tight coil
- Collagen molecules interact with each other to form fibrils through covalent bonds between adjacent amino acid R groups
- Collagen fibrils form strong bundles called collagen fibres
- Function and properties
- Flexible but with tremendous tensile strength (can withstand large pulling forces without stretching or breaking)
- Collagen fibre alignment varies depending on the forces it must endure
- In tendons, fibres align in parallel along the length of the tendon
- In skin, fibres may form layers with different directions to resist pulling forces from multiple directions
2.7 Water
- Major component of cells, typically making up 70-95% of cell mass; humans are about 60% water
- Provides an environment for organisms living in water, covering three-quarters of the planet
- Properties of water
- Water molecules are held together by hydrogen bonds, which gives water unique properties
- Water exists as a liquid at Earth temperatures due to hydrogen bonding
- Provides a medium for molecules and ions to mix, enabling life processes to occur
- Water molecules are held together by hydrogen bonds, which gives water unique properties
- Effects of hydrogen bonding
- Makes it harder to separate water molecules, which influences physical properties
- More energy required to break hydrogen bonds makes it difficult to convert water from liquid to gas
Water as a solvent
- Water is an excellent solvent for ions and polar molecules due to the attraction between water molecules
- Dissolves chemicals by separating and surrounding them
- This allows molecules or ions in solution to move freely and react with other chemicals
- Many biological processes occur in solution, making water ideal for transporting substances (e.g. in blood, lymphatic systems, xylem, phloem)
- Water and non-polar molecules
- Non-polar molecules (like lipids) are insoluble in water
- Water molecules are attracted to each other, causing non-polar molecules to cluster together
- This hydrophobic interaction is crucial in protein structure, membrane structure, and the stability of proteins and membranes
High specific heat capacity
- The heat capacity of a substance is the heat required to raise its temperature by a given amount
- The specific heat capacity of water is the heat required to raise the temperature of 1 kg of water by 1°C.
- It’s high because hydrogen bonds between water molecules resist movement, requiring more energy
- Also allows water to store more energy per temperature rise hydrogen bonding
- Biological implications
- Maintains constant cellular and body temperature for optimal reactions
- Large water bodies provide stable habitats for aquatic ecosystems (despite air temperature changes)
High latent heat of vaporization
- High latent heat of vaporization
- Latent heat of vaporization measures the heat energy required to change a liquid to a gas, such as when water evaporates.
- Water has a high latent heat of vaporization due to its strong hydrogen bonds, which require significant energy to break before the molecules can escape as gas
- This energy loss during vaporization causes cooling in the surroundings
- Cooling mechanism
- Evaporation acts as a cooling mechanism in organisms, such as sweating or panting in mammals, which helps regulate body temperature and prevent overheating
- A large amount of heat can be lost with minimal water loss, helping reduce the risk of dehydration
- In plants, evaporation during transpiration helps cool the leaves.
- Freezing resistance
- Must release a lot of energy → lower chances of freezing
- This protects aquatic habitats by reducing freezing in water bodies
- Prevent freezing in organisms with high water content
2.8 Testing for biological molecules
Reducing sugars
- Add Benedict’s reagent to the sample solution you are testing and heat it in a water bath
- Positive result: turns green/yellow/orange/red-brown
Starch
- Add iodine solution to the sample solution
- Positive result: turns blue black
Lipids
- Add absolute ethanol to a sample solution
- Shake vigorously (allows lipids to dissolve in ethanol)
- Pour solution into water
- Positive result: forms a cloudy white suspension
Proteins
- Add Biuret’s reagant to the sample solution
- Positive result: turns purple
Non-reducing sugars
- Carry out Benedict’s test on the sample solution
- If you get a negative result, start again with a fresh sample of the solution
- Heat the sample solution with hydrochloric acid
- If a non-reducing sugar is present, it will break down to monosaccharides
- Add sodium hydroxide to neutralize the solution (to ensure Benedict’s reagent works)
- Add Benedict’s reagent and heat as before and look for a positive result
