AS Level Chemistry 9701
1. Atomic Structure
Written by: Adhulan Rajkamal
Formatted by: Rithanya S
1.1 Particles in the atom
- Atoms are mostly empty space
- Very small, dense nucleus with protons and neutrons
- Electrons found in shells in the empty space around the nucleus
| Subatomic Particle | Symbol | Relative Mass | Relative Charge |
|---|---|---|---|
| Electron | e | 1/1836 | -1 |
| Neutron | n | 1 | 0 |
| Proton | p | 1 | +1 |
- Most mass in nucleus
- Nucleus is positively charged
Atomic and mass no.
- Atomic/ proton no. (Z) – number of protons in an atom
- Mass/ nucleon no. (A) – protons + neutrons in an atom
Beams of p, n and e in an electric field

Calculating no. of p, n and e in atoms and ions
- No. of p = Atomic no. (Z)
- No. of n = Mass no. (A) – Atomic no. (Z)
- No of e (neutral atom) = Atomic no. (Z)
- No of e (ions):
- Cation (+ve ion) = Protons (Z) – Charge
- Anion (-ve ion) = Protons (Z) + Charge
1.2 Isotopes
- Isotopes: Atoms of the same element with the same number of protons but different numbers of neutrons.
- Same atomic no. but different mass no
$$A^x_y-\text{x = mass/ nucleon no. | y = atomic/ proton no. | A = Element symbol}$$
- Isotopes of same element:
- Same chemical properties same no. of electrons
- Different physical properties (variation in mass and density) different number of n
1.3 Electrons, energy levels and atomic orbitals
- e– arranged in energy levels/ principal quantum shells around the nucleus
- Symbol → n
- Numbered according to distance from nucleus:
- n = 1 → closest to nucleus; energy level with lowest energy e–
- As n↑ electrons have more energy → held less tightly to nucleus
- As n↑ distance from nucleus increases
- Sub-shells: Regions of the principal quantum shells where electrons exist in defined areas associated with particular amounts of energy.
- Names (increasing energy): s < p < d < f

- Sub-shells in the order of increasing energy. The number prefix is the principal quantum number (n) – shows which shell the sub-shell belongs to.
- n = 1 → 1 sub-shell; n = 2 → 2 sub shells
- Atomic orbitals: Regions of space outside the nucleus that can be occupied by a maximum of two e–.
- Orbitals are also named → s, p, d, f
| Subshell | No. of e- | No of orbitals |
|---|---|---|
| s | 2 | 1 |
| p | 6 | 3 (Px, Py, Pz) |
| d | 10 | 5 |
- Shapes of orbitals: Probability of finding e– within these 3D shapes is high

Electronic configuration

Eg: Mg → 1s2 2s2 2p6 3s2; K → [Ar] 4s1
- [Ar] → Short-hand notation → represents the electronic config. of Argon.
- Eg (with orbital): Si → 1s² 2s² 2px² 2py² 2pz² 3s² 3px¹ 3py¹ (just for understanding)
- Note: Most stable electronic config. → lowest energy → therefore, sub-shells filled in the order of increasing in energy (refer image in previous page)
- Ground state: the lowest energy state of an atom or other particle
⚠️ Note
Subshell 4s is filled before subshell 3d!
Chromium and Copper exception
- Chromium → [Ar]4s1 3d5 instead of [Ar]4s2 3d4
- Copper → [Ar]4s1 3d10 instead of [Ar]4s2 3d9
- Half filled and fully filled sub-shells are more stable → Electrons arrange them in the most stable form.
Orbitals and the periodic table

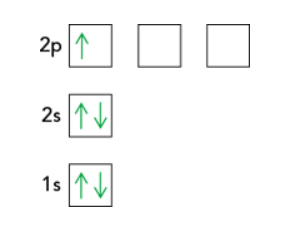
Box notation
- Each box → atomic orbital
- Arranged in increasing order of energy from bottom to top
- Arrow to represent e–
- Direction of arrow → spin of electron
- Paired electrons have opposite spin to minimise repulsion
- Electronic configuration of Boron in box notation:
- Electronic configuration of Fe in box notation (another way of representation):
- Wherever possible e– occupy separate orbital in same sub-shell to minimise repulsion (Chromium and Copper are exceptions)
⚠️ Spin-pair repulsion
- A pair of e- in the same orbital repel each other.
- This repulsion is more than that between single atoms in separate orbitals – that is why e- in the p and d sub-shells go into separate orbital before pairing up.
- Opposite spin reduces the repulsion.
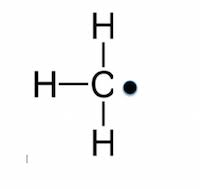
Free radical
- A species with one (or more) unpaired e–
- The unpaired e– in a free radical is shown as a dot
⚠️ Species
The word species refers to different particles such as atoms, ions, molecules, free radicals or electrons when we want to write in general terms or about more than one type of particle.
The word species refers to different particles such as atoms, ions, molecules, free radicals or electrons when we want to write in general terms or about more than one type of particle.
Electronic config. of ions
- Anions (-ve ions) have extra electrons → add extra electrons to the configuration applying the same rules
- Cations (+ve ions) have lesser electrons → remove electrons from the configuration (highest energy subshell first)
⚠️ Exceptions → d-block elements
- Although the 3d subshell is filled after the 4s subshell, electrons from the 4s subshell are lost first in d-block elements.
- In other words, despite the 3d subshell having a higher energy than the 4s, the 4s electrons are the first to be removed during ionisation in these elements.
1.4 Ionisation energy
- Ionisation → fire high speed electron at an element → knock out an electron from the element
First ionisation energy (IE1)
- ⚠️ Definition: Energy required to remove 1 mole of electrons from 1 mole of isolated gaseous atoms to form 1 mole of unipositive gaseous ions.
- Unit → kJ mol-1
- Symbol → IE1
- Eg equation: Ca(g) → Ca⁺(g) + e⁻
- Energy required → 590kJ mol-1
Successive ionisation energies
- IE1 → IE2 → IE3→ …
- Eg equations:
- IE2: Ca⁺(g) → Ca²⁺(g) + e–
- IE3: Ca²⁺(g) → Ca³⁺(g) + e–
- Successive IE show an increasing trend
- Net +ve charge on ion ↑
- More attraction b/w the proton and remaining e–
- More energy needed to overcome the force of attraction
- Big jump in IE → e– removed from principal quantum shell closer to the nucleus
Factors affecting IE
- Size of nuclear charge → more +ve charge, stronger attraction
- Generally, as proton no. (Z) increases, IE increases
- Distance of outer e– → attraction reduces rapidly with distance
- Shielding effect of inner electrons
- Full inner shells prevent outer shell from experiencing full nuclear charge
- IE ↓ as the number of full electron shells b/w nucleus and outer electrons increases
- Spin-pair repulsion → e– in the same atomic orbital repel each other more than e– in different orbitals
- Increased repulsion → easier to remove → lower IE
Variation in atomic and ionic radii
🧠 Covalent atomic radius
Half the distance between the nuclei of two atoms that are covalently bonded.
Half the distance between the nuclei of two atoms that are covalently bonded.
- Atomic radius increases down a group
- No. of shells ↑
- Shielding effect ↑
- Nuclear charge increases but not dominant factor
- Atomic radius decreases across a period
- Same shielding effect
- Nuclear charge ↑ hence pull outer-shell electrons closer
- Ionic radius shows the same trend as Atomic radius down a group and across a period and is explained by the same logic.
Patterns in ionisation energies
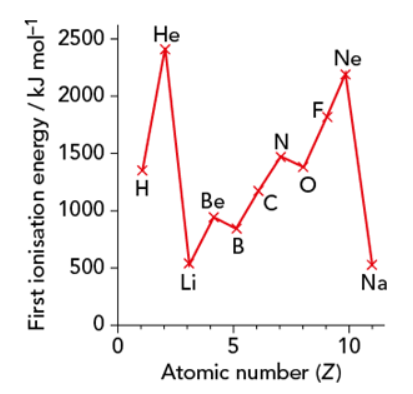
Across a period
- General increase in IE across a period
- Nuclear charge ↑
- Shielding effect constant (electrons removed from same shell)
- Rapid decrease b/w last element of one period and first element of the next
- e– removed from the next shell
- Distance b/w nucleus and outer shell electrons ↑
- Shielding effect ↑
- General increase in IE across a period
- Nuclear charge increases (but other factors are more dominant)
🧠 Exceptions
- Beryllium & Boron:
- Although Boron has a greater nuclear charge, it has a lesser IE1 than Beryllium
- Beryllium → 1s2 2s2
- Boron → 1s2 2s2 2p1
- 2p is slightly farther away from nucleus than 2s → slight increase in shielding effect → outweigh nuclear charge
- Inner sub-shells also cause shielding effect
- Although Boron has a greater nuclear charge, it has a lesser IE1 than Beryllium
- Nitrogen & Oxygen:
- Electron removed from the same 2p subshell
- But Oxygen has paired e– → spin-pair repulsion → easier to remove
Down a group
- IE1 decreases as you go down a group
- Distance b/w nucleus and outer shell electrons increases
- Shielding effect increases
- This outweighs the increase in nuclear charge