AS Level Biology 9700
4. Cell membranes and transport
Written by: Rhia Sakthivel
Formatted by: Pranav I
Index
4.1 Fluid mosaic membranes
- Phospholipids form membranes around cells and organelles
- Phospholipids have hydrophilic (polar) heads and hydrophobic (non-polar) tails
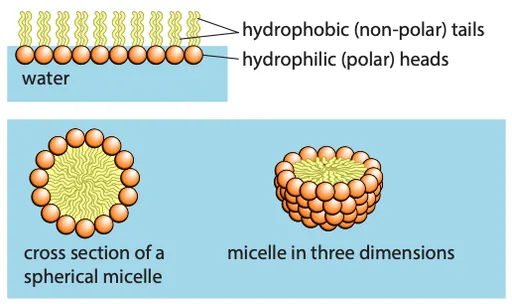
- In water, phospholipids form:
- Micelles: Hydrophilic heads face outward, shielding hydrophobic tails
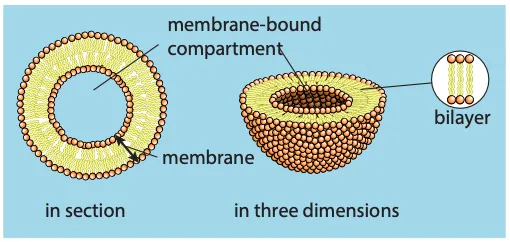
- Bilayers: Hydrophobic tails are shielded by hydrophilic heads, forming a membrane
- The phospholipid bilayer is the basic structure of membranes, about 7 nm wide
- Membranes also contain proteins, visible in electron micrographs
Fluid mosaic model
- Fluid: phospholipids and proteins move by diffusion (similar to the fluidity of olive oil)
- Phospholipid molecules move sideways in their layers
- Some proteins move within the bilayer, while others remain fixed to structures
- Mosaic: describes the pattern formed by scattered protein molecules when viewed from above
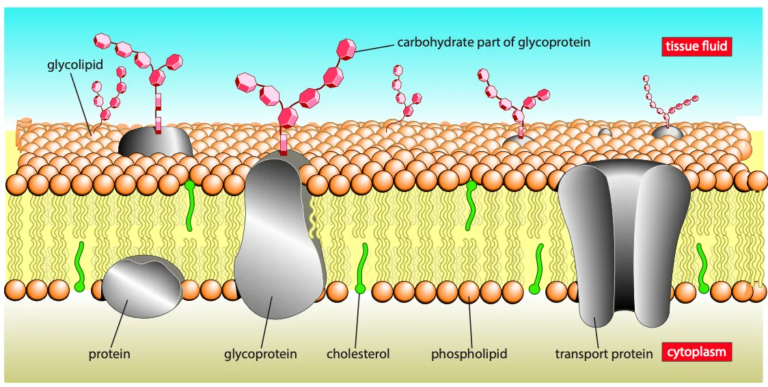
Cholesterol, glycolipids and glycoproteins
- The membrane consists of a bilayer of phospholipids with hydrophobic tails facing inward and hydrophilic heads facing outward into the aqueous environment
- Proteins:
- Found in the inner or outer layer or spanning the whole membrane (transmembrane proteins)
- Proteins have both hydrophobic (non-polar) and hydrophilic (polar) regions
- Hydrophobic regions interact with phospholipid tails while hydrophilic regions interact with the aqueous environment or form pores for the passage of polar substances
- Glycoproteins and glycolipids:
- Many proteins and lipids have short carbohydrate chains
- Found on the outer surface of cell membranes
- Made in the Golgi body
- Cholesterol is also present in the membrane
4.2 Roles of the molecules found in the membranes
Phospholipids
- Form a bilayer, which is the basic structure of the membrane
- Fluidity:
- Affected by the saturation of phospholipid tails (unsaturated tails increase fluidity)
- Longer tails reduce fluidity
- Temperature decrease makes membranes less fluid as phospholipid molecules get close together
- Organisms like bacteria and yeasts respond by increasing unsaturated fatty acids in their membranes
- Hydrophobic tails:
- Create a hydrophobic core
- Make it difficult for polar molecules or ions to pass through the membrane
- Membranes act as a barrier to most water-soluble substances
- Preventing leakage of sugars, amino acids, and proteins out of the cell
- Blocking unwanted molecules from entering
- Some phospholipids can be modified to act as signaling molecules
Cholesterol
- A small molecule with hydrophilic heads and hydrophobic tails
- Fits between phospholipid molecules, with heads at the membrane surface
- Found in animal cell membranes in amounts similar to phospholipids
- Less common in plant cell membranes and absent in prokaryotes (where similar compounds serve the same function)
- Functions:
- Provides mechanical stability to membranes by reducing fluidity
- Prevents membranes from breaking and cells from bursting
- The hydrophobic regions help block ions and polar molecules from passing through
- Important in the myelin sheath of nerve cells to prevent ion leakage, ensuring efficient nerve impulse transmission
- At low temperatures, it prevents phospholipid tails from packing too closely, maintaining correct membrane fluidity for cell survival in cold conditions
Glycolipids, glycoproteins and proteins
- Receptor molecules:
- Carbohydrate chains help glycoproteins and glycolipids act as receptors
- Bind with specific substances (such as hormones or neurotransmitters) to trigger internal cell reactions
- Example: glucagon receptor in liver cells
- Cell-to-cell recognition:
- Glycolipids and glycoproteins serve as cell markers or antigens
- Enable cells to recognize each other (e.g. ABO blood group antigens)
- Transport proteins:
- Provide channels or passageways for ions and polar molecules to pass through
- Include channel proteins and carrier proteins, specific for different ions or molecules
- Enzymes:
- Some membrane proteins act as enzymes (e.g. digestive enzymes in small intestine cells)
- Catalyze reactions (hydrolysis of disaccharides)
- Cytoskeleton:
- Proteins attached to cytoskeleton filaments help maintain cell shape and assist in movement
- Other roles:
- In organelles like mitochondria and chloroplasts, membrane proteins are involved in respiration and photosynthesis
4.3 Cell signaling
✅ Signaling (definition)
The process of transmitting a message from one place to another in an organism
- It is essential for coordinating cellular activities and responding to environmental stimuli
- Signaling pathways can be electrical (e.g. nervous system) or chemical (e.g. hormone system)
Chemical signaling pathways
- Stimulus: a stimulus causes cells to secrete a specific chemical, called a ligand (e.g. glucagon in response to low blood sugar)
- Transport: the ligand is transported to the target cells, typically through the bloodstream
- Binding: the ligand binds to specific cell surface receptors on the target cells (glycolipids, glycoproteins or proteins)
- Transduction: the binding changes the shape of the receptor, triggering a cascade of reactions inside the cell
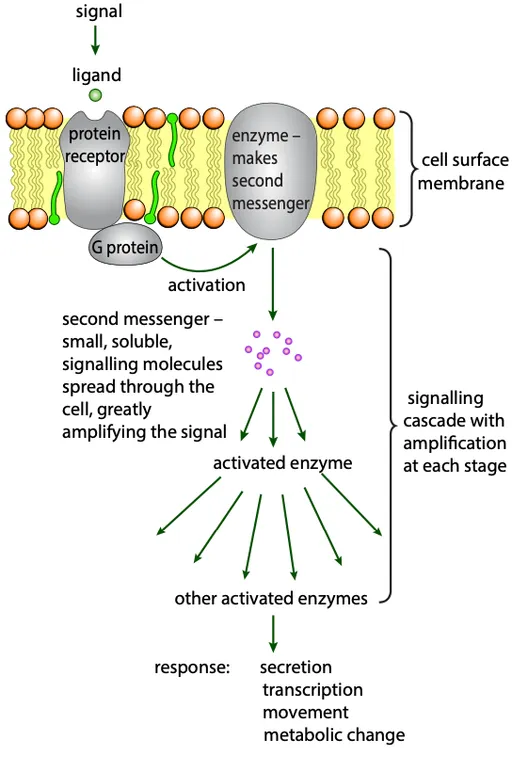
G proteins and second messengers
- The receptor interacts with a G protein, which activates the release of a second messenger
- Amplification: one receptor activation leads to the production of many second messenger molecules, amplifying the signal
- Enzyme activation: second messengers activate enzymes, which further propagate the signal, eventually leading to changes in cell metabolism
- This series of events is called a signaling cascade
Alternative pathways for receptors
- Ion channel: some receptors open ion channels, altering the membrane potential
- Enzyme: some receptors act as membrane-bound enzymes, triggering metabolic changes
- Intracellular receptor: some signals (e.g. steroid hormones) pass through the membrane and bind to intracellular receptors, directly altering gene expression
🚨 Some signaling molecules are hydrophobic and can diffuse across the cell membrane
🚨 Cells can signal each other through direct contact (e.g. lymphocytes recognizing foreign antigens)
4.4 Movement into and out of cells
Simple diffusion
✅ Diffusion (definition)
The net movement of molecules or ions from a region of higher concentration to a region of lower concentration due to random molecular motion
- Molecules move down a concentration gradient until equilibrium is reached (when molecules are evenly spread)
- Random movement occurs due to the kinetic energy of molecules or ions
- Molecules that can diffuse through cell membranes:
- Respiratory gases (oxygen and carbon dioxide) → uncharged and non-polar
- Water molecules → polar but small enough to diffuse rapidly across the bilayer
- Hydrophobic molecules → pass through the membrane’s hydrophobic interior
- Factors affecting diffusion rate:
- Steepness of the concentration gradient
- Temperature
- Nature of molecules or ions (e.g. size, polarity)
- Surface area of the membrane
Steepness of the concentratin gradient
- The gradient is the difference in substance concentration across the membrane
- A steeper gradient results in a faster diffusion rate
- Net movement occurs
- Molecules move in both directions, but more move from higher to lower concentration
- The direction of net movement depends on the concentration gradient
Temperature
- High temperatures increase the kinetic energy of molecules and ions
- Faster-moving molecules and ions result in a higher rate of diffusion
Nature of molecules or ions
- Size
- Large molecules diffuse more slowly as they require more energy to move
- Small molecules diffuse faster due to lower energy requirements
- Polarity
- Non-polar molecules (e.g. glycerol, alcohol, steroid hormones) diffuse easily through cell membranes as they are soluble in the non-polar interior
- Polar molecules diffuse less easily through cell membranes
Surface area of the membrane
- A larger surface area allows more molecules or ions to cross at once, increasing the rate of diffusion
- Cell membranes increase surface area through folding (e.g. microvilli in intestinal cells, cristae in mitochondria)
- Volume increases more rapidly than surface area as size increases
- Larger cells have a smaller surface area-to-volume ratio
- Diffusion is effective only over short distances, limiting cell size
- Molecules like amino acids diffuse micrometers in seconds but take hours to diffuse centimeters
- Most eukaryotic cells are under 50 micrometers in diameter; prokaryotic cells are even smaller
- Large aerobic cells would quickly deplete oxygen and die due to inefficient diffusion
Facilitated diffusion
✅ Facilitated diffusion (definition)
The diffusion of a substance through a transport protein (carrier protein or channel protein) across a cell membrane
- Large polar molecules (e.g. glucose, amino acids) cannot diffuse through the phospholipid bilayer
- Ions (e.g. Na⁺ and Cl⁻) also cannot pass through the bilayer
- Facilitated diffusion allows these molecules to cross the membrane with the help of transport proteins
- DOES NOT require energy from ATP
- Channel and carrier proteins are the two types of transport proteins involved in facilitated diffusion
- Each transport protein is highly specific, allowing only one type of molecule or ion to pass through
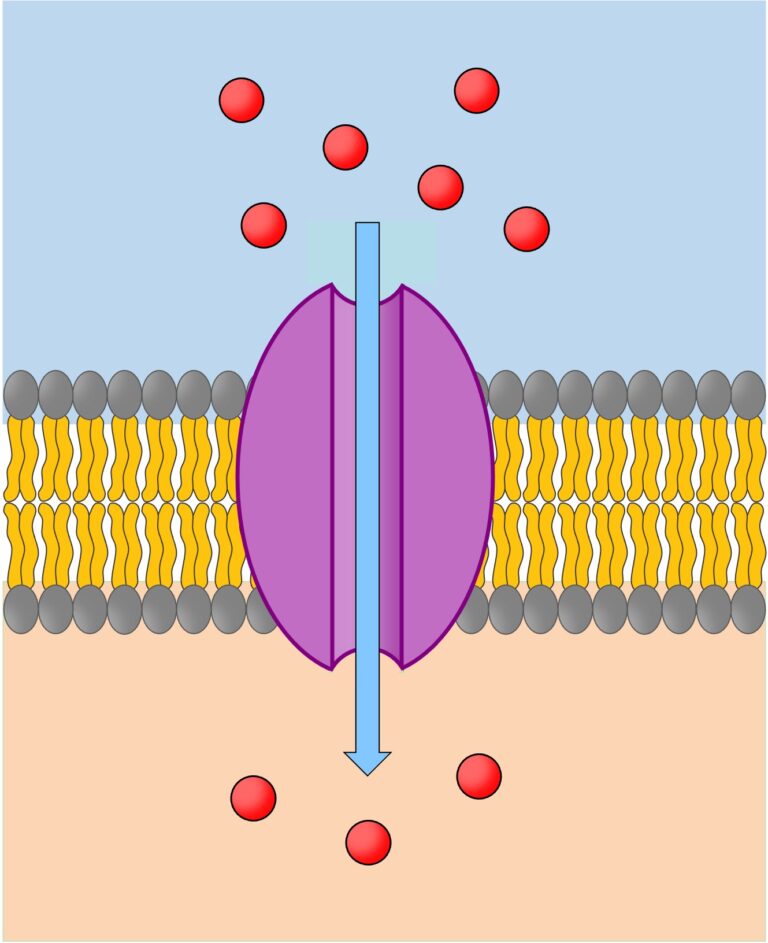
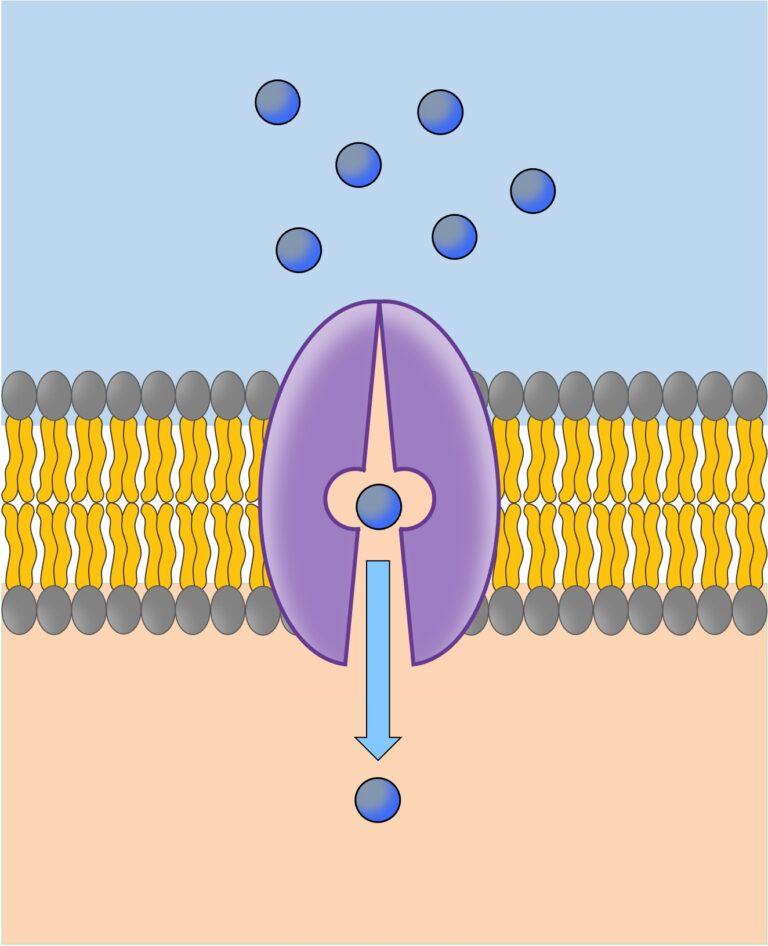
Channel proteins
- Have a fixed shape
- Have water-filled pores, enabling charged substances like ions to diffuse through the membrane
- Gated → they can open or close to regulate ion exchange
- Gated proteins control ion movement
- Sodium ion (Na⁺) entry during action potential production
- Potassium ion (K⁺) exit during repolarization
- Some channels are formed by a single protein, while others are made from multiple proteins combined
- Channel proteins involved in facilitated diffusion do not require energy from ATP
Carrier proteins
- Can flip between two shapes
- Alternate the binding site’s orientation to either side of the membrane, enabling molecule or ion transport
- Carrier proteins involved in facilitated diffusion change shape spontaneously
- Pumps (a type of carrier protein) that require energy (ATP) and are involved in active transport
Rate of diffusion through channel and carrier proteins
- The rate of facilitated diffusion depends on the number of channel or carrier proteins in the membrane
- For channel proteins, the rate is also influenced by whether they are open or closed
Osmosis
✅ Osmosis (definition)
The net diffusion of water molecules from a region of higher water potential to a region of lower water potential, through a partially permeable membrane
- Osmosis is a type of diffusion that involves only water molecules
- Solute + solvent = solution
- A partially permeable membrane allows only certain molecules (like water) to pass through
- In a comparison of two solutions separated by a membrane:
- The solution with a higher solute concentration is more concentrated
- The solution with a lower solute concentration is more dilute
- Without the membrane, solute and water molecules would move randomly, spreading evenly
- With the membrane, solute molecules cannot pass through, only water molecules can
- Water molecules move from a region of higher water potential to a region of lower water potential
- Over time, water molecules spread out evenly, causing the volume of the more concentrated solution to increase
- This process is called osmosis
Water potential
- Water potential (ψ) refers to the tendency of water to move from one place to another
- Water always moves from a region of higher water potential to a region of lower water potential, down a water potential gradient
- Water potential reaches equilibrium when it is the same throughout the system
- Water potential depends on two factors:
- Concentration of the solution
- Pressure applied to the solution
- A dilute solution has a higher water potential than a concentrated solution
- Applying pressure to a solution increases its water potential, making it higher than the same solution with no pressure
Measuring water potential
- Water potential is measured in kilopascals (kPa)
- The water potential of pure water is 0 kPa
- Solutions have a lower water potential than pure water, so their water potential is negative
- A dilute solution has a less negative water potential than a concentrated solution
- E.g. a solution with a water potential of -10 kPa has a higher water potential than one with -20 kPa
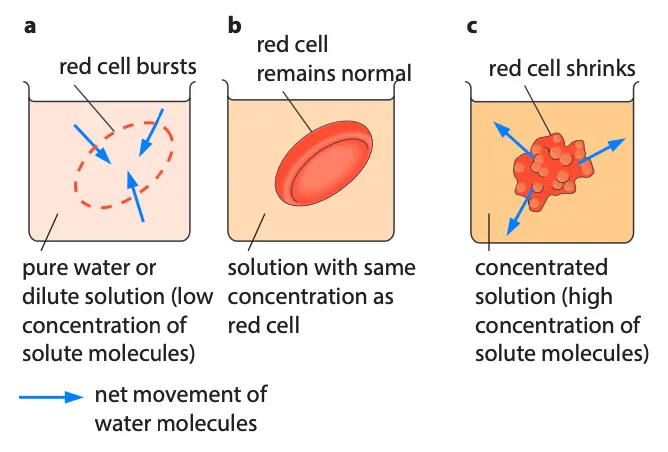
Osmosis in animal cells
- Red blood cells are commonly used to study osmosis in animal cells
- If the water potential of the surrounding solution is too high, the cell swells and bursts
- If the water potential is too low, the cell shrinks
- Maintaining a constant water potential inside animal bodies is important to prevent these effects
Osmosis in plant cells
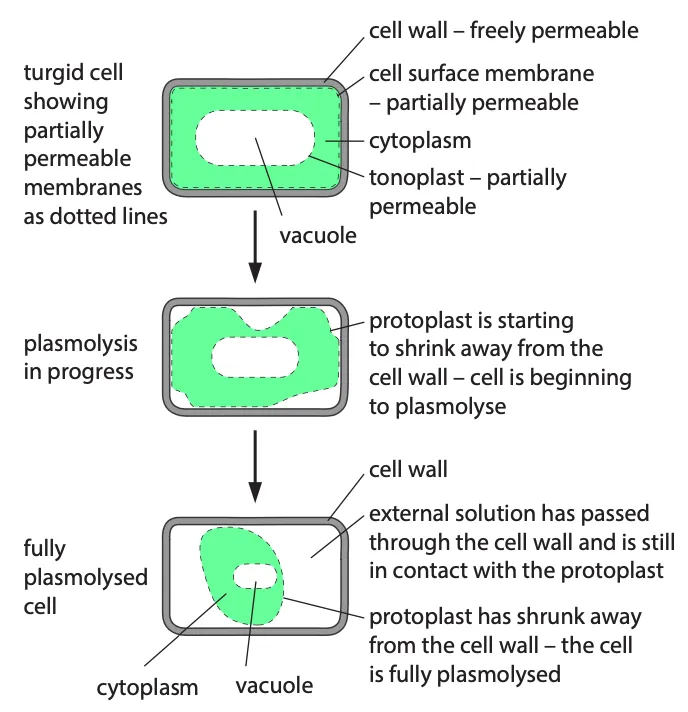
- Plant cells have cell walls that are rigid and prevent bursting
- When placed in a solution with higher water potential, water enters the cell by osmosis, causing the protoplast to expand
- The cell wall resists expansion, building up internal pressure, and increasing the water potential until equilibrium is reached
- A fully inflated plant cell is described as turgid
- In a solution with lower water potential, water leaves the plant cell, causing the protoplast to shrink
- As the protoplast shrinks, it pulls away from the cell wall, a process called plasmolysis
- The point at which plasmolysis begins is called incipient plasmolysis, where the protoplast exerts no pressure on the cell wall
- Equilibrium is reached when the water potential of the cell equals that of the external solution
- Plasmolysis can be observed with a microscope using epidermal strips from plants like rhubarb or onion bulbs
Active transport
✅ Active transport (definition)
The movement of molecules or ions through transport proteins across a cell membrane, against their concentration gradient, using energy from ATP
- Some ions (like potassium and chloride) are more concentrated inside cells than outside
- Active transport is responsible for maintaining this concentration gradient by moving ions against the gradient
- Active transport requires energy (usually provided by ATP) to move ions or molecules from low to high concentration
- Pumps (carrier proteins) are used for active transport and are specific to certain molecules or ions
- E.g. sodium-potassium pump → pumps 3 sodium ions out and 2 potassium ions in per ATP molecule used
- Active transport plays a role in kidney reabsorption, digestion absorption, and transport in plants
- Active transport requires ATP from cell respiration and can occur either into or out of the cell
Endocytosis
- Requires energy in the form of ATP
- Larger scale than previous mechanisms → bulk transport
- In endocytosis, the cell surface membrane engulfs material to form a small sac
- Phagocytosis (cell eating): this is the bulk uptake of solid material
- Phagocytes are cells that ingest and destroy pathogens or damaged body cells
- The vacuoles formed are called phagocytic vacuoles
- Pinocytosis (cell drinking): this is the bulk uptake of liquid
- The vacuoles (or vesicles) formed are often extremely small
- In this case, the process is called micropinocytosis
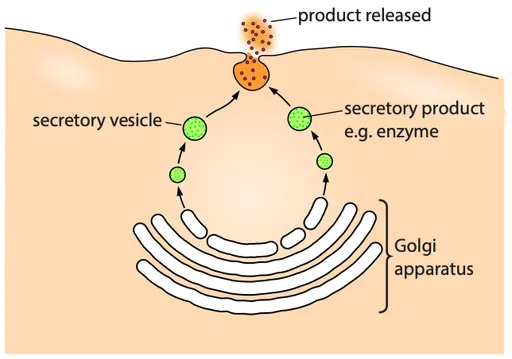
Exocytosis
- Exocytosis is the reverse of endocytosis and involves the removal of materials from cells
- E.g. secretion of digestive enzymes from pancreatic cells
- Requires energy in the form of ATP
- It is a form of bulk transport
- Secretory vesicles pinch off from the Golgi apparatus and carry materials through the cytoplasm to the cell surface for release
- The vesicles fuse with the cell membrane and then open up so that the materials are released out of the cell
- Plant cells use exocytosis to transport cell wall-building materials outside the cell