AS Level Biology 9700
3. Enzymes
Written by: Pranav I
Formatted by: Pranav I
Formatted by: Pranav I
Index
3.1 What is an enzyme?
-
Enzymes are globular proteins that catalyse reactions inside cells (intracellular enzymes), or are secreted to catalyse reactions outside cells (extracellular enzymes)
- Enzymes are globular proteins that catalyse reactions inside cells (intracellular enzymes), or are secreted to catalyse reactions outside cells (extracellular enzymes)
3.2 Mode of action of enzymes
The lock-and-key hypothesis and the induced-fit hypothesis
- Active site: An area on an enzyme molecule where the substrate can bind
- Random movement of enzyme and substrate brings the substrate into the active site
-
Lock-and-key hypothesis: A hypothesis for enzyme action which suggests that the substrate is a complementary shape to the active site of the enzyme and fits exactly into the site; the enzyme shows specificity for the substrate
- The substrate is held in place by temporary bonds with some of the R groups of the enzyme’s amino acids → enzyme-substrate complex
- Each enzyme will act on only one type of substrate molecule
-
Induced-fit hypothesis: A hypothesis for enzyme action which suggest that the substrate is a complementary shape to the active site of the enzyme, but not an exact fit – the enzyme, or sometimes the substrate, can change shape slightly to ensure a perfect fit, but it is still described as showing specificity
- Makes catalysis even more efficient
- An enzyme-product complex is briefly formed once the reaction is over
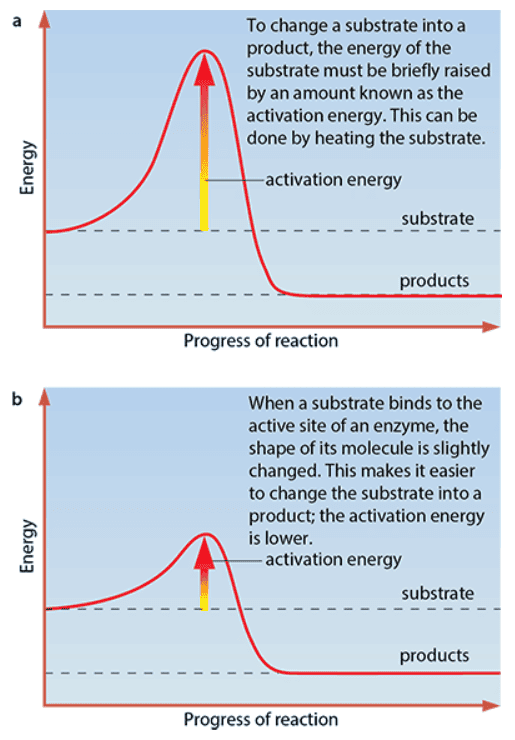
- Enzymes increase the rate at which chemical reactions occur by lowering activation energy (the energy that must be provided to make a reaction take place)
- Active site: An area on an enzyme molecule where the substrate can bind
- Random movement of enzyme and substrate brings the substrate into the active site
-
Lock-and-key hypothesis: A hypothesis for enzyme action which suggests that the substrate is a complementary shape to the active site of the enzyme and fits exactly into the site; the enzyme shows specificity for the substrate
- The substrate is held in place by temporary bonds with some of the R groups of the enzyme’s amino acids → enzyme-substrate complex
- Each enzyme will act on only one type of substrate molecule
-
Induced-fit hypothesis: A hypothesis for enzyme action which suggest that the substrate is a complementary shape to the active site of the enzyme, but not an exact fit – the enzyme, or sometimes the substrate, can change shape slightly to ensure a perfect fit, but it is still described as showing specificity
- Makes catalysis even more efficient
- An enzyme-product complex is briefly formed once the reaction is over
- Enzymes increase the rate at which chemical reactions occur by lowering activation energy (the energy that must be provided to make a reaction take place)
3.3 Investigating the progress of an enzyme-catalyzed reaction
Measuring the rate of formation of products
- Catalase catalyzes the breakdown of H2O2 to water and oxygen
- The oxygen (product) released can be collected and measured to follow the reaction
- The rate of an enzyme-catalyzed reaction is always fastest at the beginning (initial rate of reaction)
Measuring the rate of disappearance of substrate
- A colorimeter can be used to measure the progress of an enzyme-catalysed reaction that involves colour change
- Colorimeter: An instrument that measures the colour of a solution by measuring the absorption of different wavelengths of light
- Amylase breaks down starch to maltose
-
Colour of the reaction solution samples will change from blue-black to pale brown (when tested with iodine solution) as the concentration of starch in the reaction mixture decreases
- The colour acts as a measure of the amount of starch remaining
3.4 Factors that affect enzyme action
The effect of temperature on the rate of enzyme action
- At low temperatures, the kinetic energy of molecules is relatively low
- As temperature rises, the kinetic energy of molecules increases and they move faster
- Collisions happen more frequently and with more energy → easier for the reaction to occur
- Above a certain temperature, the enzyme molecules denature as bonds holding their 3D structure begin to break due to rapid vibrations
- The active site loses its shape
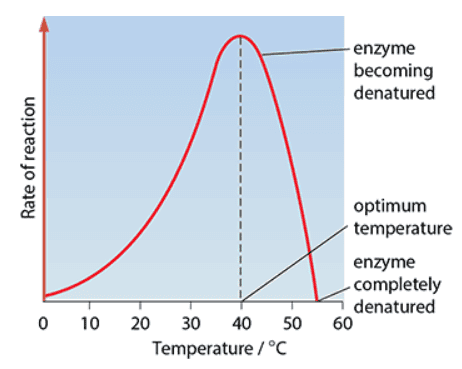
- Optimum temperature: Temperature at which an enzyme catalyses a reaction at the maximum rate
- At low temperatures, the kinetic energy of molecules is relatively low
- As temperature rises, the kinetic energy of molecules increases and they move faster
- Collisions happen more frequently and with more energy → easier for the reaction to occur
- Above a certain temperature, the enzyme molecules denature as bonds holding their 3D structure begin to break due to rapid vibrations
- The active site loses its shape
- Optimum temperature: Temperature at which an enzyme catalyses a reaction at the maximum rate
The effect of pH on the rate of enzyme activity
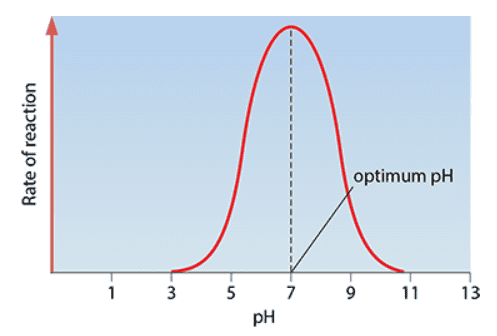
- Optimum pH: pH at which the enzyme catalyses a reaction at the maximum rate
- The optimum pH is mostly 7 but sometimes different
-
A pH which is different from the optimum oH can cause denaturation of the enzyme
- Hydrogen ions can interact with charged R groups of amino acids
- Might break the ionic bonding between R groups and affect the 3D structure of the enzyme molecule
- Might change the shape of the active site
- Buffer solutions can be used for investigations → maintain a specific pH value
- Optimum pH: pH at which the enzyme catalyses a reaction at the maximum rate
- The optimum pH is mostly 7 but sometimes different
-
A pH which is different from the optimum oH can cause denaturation of the enzyme
- Hydrogen ions can interact with charged R groups of amino acids
- Might break the ionic bonding between R groups and affect the 3D structure of the enzyme molecule
- Might change the shape of the active site
- Buffer solutions can be used for investigations → maintain a specific pH value
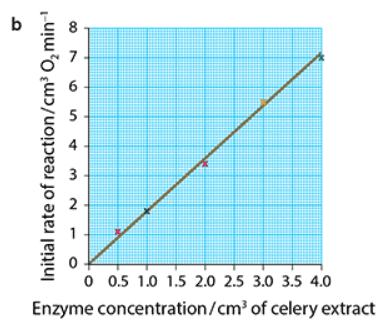
The effect of enzyme concentration on the rate of enzyme activity
- The rate of enzyme activity is directly proportional to the enzyme concentration as long as substrate concentration is high enough
- The rate of enzyme activity is directly proportional to the enzyme concentration as long as substrate concentration is high enough
The effect of substrate concentration on the rate of enzyme activity
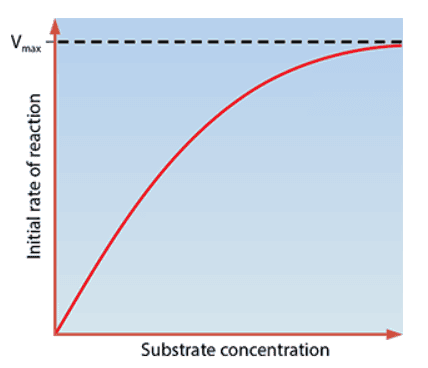
- The rate of enzyme activity increases as substrate concentration increases
- There comes a point where every enzyme active site is full → substrate molecules are queuing up for an active site to become vacant
3.5 Comparing enzyme affinities
-
Affinity is the measure of the strength of attraction between the enzyme and its substrate
- High affinity → higher likelihood of product formation when a substrate enters the active site
- Low affinity → substrate might leave the active site before the reaction takes place
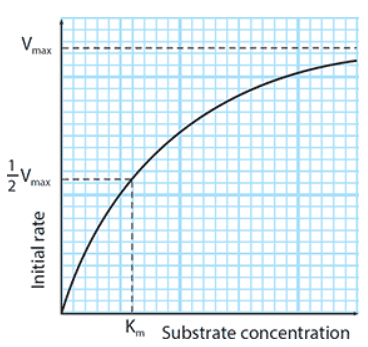
- Vmax: The theoretical maximum rate of an enzyme-catalysed reaction, obtained when all the active sites of the enzyme are occupied
-
The initial rate against substrate concentration curve never completely flattens out in practice (Vmax cannot be read off of it)
- It is possible to calculate ½ Vmax from this graph?
- Just as useful as Vmax as an indicator of how fast an enzyme works (half the enzymes’ active sites are occupied)
-
Km (Michaelis-Menten constant): The substrate concentration at which an enzyme works at half its maximum rate (½ Vmax), used as a measure of the efficiency of an enzyme; the lower the value of Km, the more efficient the enzyme
- Measure of the enzyme’s affinity for its substrate
- Higher the affinity of an enzyme to its substrate, the lower its Km will be
3.6 Enzyme inhibitors
Competitive, reversible inhibition
-
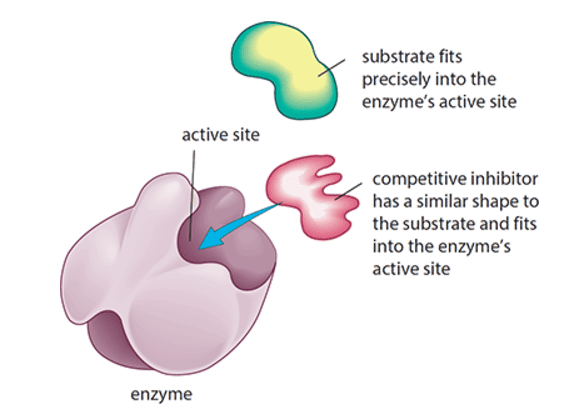
When a substance reduces the rate of activity of an enzyme by competing with the substrate molecules for the enzyme’s active site
- Increasing substrate concentration reduces the degree of inhibition
- Increasing inhibitor concentration increases the degree of inhibition
- Can be reversed by increasing substrate concentration
- Inhibitor molecules bind only briefly to the site and come out again
- Example: ethanol used as a competitive inhibitor to reduce the activity of the enzyme that converts ethylene glycol to oxalic acid after accidental consumption of ethylene glycol
Non-competitive, reversible inhibition
-
When a substance reduces the rate of activity of an enzyme, but increasing the concentration of substrate does not reduce the degree of inhibition
- Many non-competitive inhibitors bind to areas of the enzyme molecules other than the active site itself
- The inhibitor affects the normal arrangement of hydrogen bonds and hydrophobic interactions, changing the shape of the active site
- Substrate cannot bind to the active site
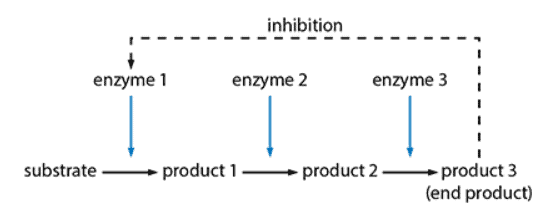
- End product is a non-competitive, reversible inhibitor of an enzyme at the beginning of the chain of reactions
- Enzyme slows down as end product concentration increases
- End product can lose its attachment to the enzyme to restore enzyme activity
3.7 Immobilizing enzymes
- Enzymes have many commercial applications and are expensive
-
Immobilised enzymes: Enzymes that have been fixed to a surface or trapped inside beads of agar gel
- Prevents them from diffusing freely in solution
-
Advantages:
- Enzymes can be reused
- Product is enzyme-free
- Tolerant of temperature and pH changes as they are held firmly in shape by the alginate and some parts are not exposed to the changes → do not denature easily
- Helps keep costs down
